Chemistry:Dichlorotetrakis(pyridine)iron(II)
From HandWiki
| |
| Names | |
|---|---|
| Other names
Tetra(pyridine)iron dichloride
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C20H20Cl2FeN4 | |
| Molar mass | 443.15 g·mol−1 |
| Appearance | yellow solid |
| Density | 1.351 g/cm3 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
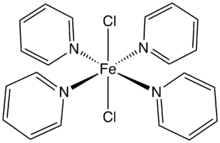
Dichlorotetrakis(pyridine)iron(II) is the coordination complex with the formula FeCl2(pyridine)4. A yellow solid, it is a prominent example of a transition metal pyridine complex. It is used as an anhydrous precursor to other iron complexes and catalysts.[1] According to X-ray crystallography, the chloride ligands are mutually trans. The complex has a high spin configuration. A monohydrate as well as several related complexes are known, e.g. CoCl2(pyridine)4 and NiCl2(pyridine)4.[2] It is prepared by treating ferrous chloride with an excess of pyridine.[3]
References
- ↑ Wu, Jessica Y.; Stanzl, Benjamin N.; Ritter, Tobias (2010). "A Strategy for the Synthesis of Well-Defined Iron Catalysts and Application to Regioselective Diene Hydrosilylation". Journal of the American Chemical Society 132 (38): 13214–13216. doi:10.1021/ja106853y. PMID 20809631.
- ↑ Long, Gary J.; Clarke, Peter J. (1978). "Crystal and molecular structures of trans-tetrakis(pyridine)dichloroiron(II), -nickel(II), and -cobalt(II) and trans-tetrakis(pyridine)dichloroiron(II) monohydrate". Inorganic Chemistry 17 (6): 1394–1401. doi:10.1021/ic50184a002.
- ↑ Baudisch, Oskar; Hartung, Walter H. (1939). "Tetrapyridino-Ferrous Chloride (Yellow Salt)". Inorganic Syntheses 1: 184–185. doi:10.1002/9780470132326.ch64.
 |

