Chemistry:Dicyclohexylurea
From HandWiki
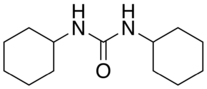
| |
| Names | |
|---|---|
| Preferred IUPAC name
N,N′-Dicyclohexylurea | |
| Other names
DCU
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C13H24N2O | |
| Molar mass | 224.348 g·mol−1 |
| Melting point | 230 to 233 °C (446 to 451 °F; 503 to 506 K) |
| Hazards | |
| Safety data sheet | External MSDS |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302 | |
| P264, P270, P301+312, P330, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Dicyclohexylurea is an organic compound, specifically, a urea. It is the byproduct of the reaction of dicyclohexylcarbodiimide with amines or alcohols. It may be prepared by the reaction of cyclohexylamine and S,S-dimethyl dithiocarbonate.[1] 1,3-Dicyclohexyl urea (DCU) is a potent soluble epoxide hydrolase (sEH) inhibitor. It has been shown to lower systemic blood pressure by 22 ± 4 mmHg in SHR.[2]
References
- ↑ Man-kit Leung; Jun-Liang Lai; King-Hang Lau; Hsiao-hua Yu; Hsiang-Ju Hsiao (1996). "S,S-Dimethyl Dithiocarbonate: A Convenient Reagent for the Synthesis of Symmetrical and Unsymmetrical Ureas". The Journal of Organic Chemistry 61 (12): 4175–4179. doi:10.1021/jo9522825. PMID 11667305. http://ntur.lib.ntu.edu.tw/bitstream/246246/164433/1/04.pdf.
- ↑ Sarbani Ghosh, Po-Chang Chiang, Jan L. Wahlstrom, Hideji Fujiwara, Jon G. Selbo andSteven L. Roberds (2008). "Oral Delivery of 1,3-Dicyclohexylurea Nanosuspension Enhances Exposure and Lowers Blood Pressure in Hypertensive Rats". Basic & Clinical Pharmacology & Toxicology 102 (5): 453–458. doi:10.1111/j.1742-7843.2008.00213.x. PMID 18312493.
 |
