Chemistry:Dihydrocaffeic acid
From HandWiki
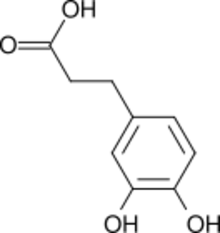
| |
| Names | |
|---|---|
| Preferred IUPAC name
3-(3,4-Dihydroxyphenyl)propanoic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| 2213449 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| 482169 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H10O4 | |
| Melting point | 136 °C (277 °F; 409 K) |
| 42.8 g/L | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
3(3,4-dihydroxy-phenyl) propionic acid commonly referred to as dihydrocaffeic acid or DHCA[1] is a phytochemical found in grapes and other plants. DHCA is known to lower IL-6 production through down regulation of DNMT1 expression and inhibition of DNA methylation of the IL-6 gene in mice. DHCA in combination with malvidin-3′-O-glucoside (Mal-gluc), is effective in promoting resilience against stress by modulating brain synaptic plasticity and peripheral inflammation. DHCA/Mal-gluc also significantly lowered depression like phenotypes in mice that had increased peripheral inflammation caused by transplantation of hematopoietic progenitor cells from other more stress-susceptible mice.[2]
References
- ↑ PubChem. "3-(3,4-Dihydroxyphenyl)propionic acid" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/348154.
- ↑ Wang, Jun; Hodes, Georgia E.; Zhang, Hongxing; Zhang, Song; Zhao, Wei; Golden, Sam A.; Bi, Weina; Menard, Caroline et al. (2018-02-02). "Epigenetic modulation of inflammation and synaptic plasticity promotes resilience against stress in mice". Nature Communications 9 (1): 477. doi:10.1038/s41467-017-02794-5. ISSN 2041-1723. PMID 29396460.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
 |

