Chemistry:Dihydropteroate
From HandWiki
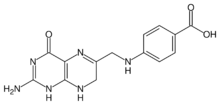
| |
| Names | |
|---|---|
| IUPAC name
4-{[(2-amino-4-oxo-1,4,7,8-tetrahydropteridin-6-yl)methyl]amino}benzoic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1226443 | |
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C14H14N6O3 | |
| Molar mass | 314.3 g/mol |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | danger |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dihydropteroate is an important intermediate in folate synthesis. It is a pterin created from para-aminobenzoic acid (PABA) by the enzyme dihydropteroate synthase.[2]
Bacteriostatic agents such as sulfonamides target dihydropteroate synthetase. The effect of dihydropteroate synthetase inhibition is comparable to that of dihydrofolate reductase inhibition by trimethoprim, another bacteriostatic agent. Combinations of these two drug types, such as the combination trimethoprim/sulfamethoxazole (TMP-SMX]), are commonly used to treat recurrent urinary tract, Shigella, Salmonella, and Pneumocystis jivoreci infections.
See also
References
- ↑ GHS: GESTIS 492946
- ↑ Hevener, Kirk E; Yun, Mi-Kyung; Qi, Jianjun; Kerr, Iain D; Babaoglu, Kerim; Hurdle, Julian G; Balakrishna, Kanya; White, Stephen W et al. (2010). "Structural Studies of Pterin-Based Inhibitors of Dihydropteroate Synthase". Journal of Medicinal Chemistry 53 (1): 166–177. doi:10.1021/jm900861d. PMID 19899766.
 |


