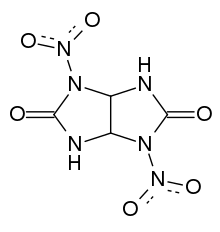
Chemistry:Dinitroglycoluril
| |
| Names | |
|---|---|
| IUPAC name
3,6-dinitro-1,3a,4,6a-tetrahydroimidazo[4,5-d]imidazole-2,5-dione
| |
| Other names
DNGU
1,4-dinitroglycoluril | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
| MeSH | 1,4-dinitroglycoluril |
PubChem CID
|
|
| UN number | 0489 |
| |
| |
| Properties | |
| C4H4N6O6 | |
| Molar mass | 232.112 g·mol−1 |
| Density | 1.94 g/cm3[1] |
| Boiling point | 252.87 °C (explosive decomposition) |
| Thermochemistry | |
Std molar
entropy (S |
128.4 J/(mol·K)[1] |
Std enthalpy of
formation (ΔfH⦵298) |
-74 kcal/mol[2] |
| Explosive data | |
| Detonation velocity | 8450 m/s[1] |
| Related compounds | |
Related compounds
|
Glycoluril |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dinitroglycoluril (DNGU) is a high explosive[1] chemical compound with the formula C4H4N6O6. Dinitroglycoluril is of growing interest due to its stability, ability to mix with oxygen positive explosives to form composites, and it is a precursor to tetranitroglycoluril.[2]
Preparation and decomposition
Dinitroglycoluril can be created by nitrating glycoluril with concentrated nitric acid.[3]
- C
4H
6N
4O
2 + 2 HNO
3 → C
4H
4N
6O
6 + 2 H
2O
The activation energy required to begin decomposition of dinitroglycoluril is 165 kJ/mol.[2] When dinitroglycoluril is heated to 243 °C in an inert atmosphere, the two nitrate groups break off and the two central carbon atoms form a double bond.[1]
Sensitivity
The impact sensitivity of dinitroglycoluril was determined using the Bruceton-staircase procedure, which found a h50 of 88 cm. Friction sensitivity was determined by a Julius-Peters apparatus, which found a sensitivity of 25 kg.[2]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Zhao, Feng-Qi; Rong-Zu, Hu; Chen, Pei; Luo, Yang; Gao, Sheng-Li; Song, Ji-Rong; Shi, Qi-Zhen (2010-08-26). "Kinetics and mechanism of the exothermic first-stage decomposition reaction of dinitroglycoluril" (in en). Chinese Journal of Chemistry 22 (7): 649–652. doi:10.1002/cjoc.20040220707. https://onlinelibrary.wiley.com/doi/10.1002/cjoc.20040220707.
- ↑ 2.0 2.1 2.2 2.3 Khire, V; Talawar, M; Prabhakaran, K; Mukundan, T; Kurian, E (2005-03-17). "Spectro-thermal decomposition study of 1,4-dinitroglycoluril (DINGU)" (in en). Journal of Hazardous Materials 119 (1–3): 63–68. doi:10.1016/j.jhazmat.2004.12.020. PMID 15752849. https://linkinghub.elsevier.com/retrieve/pii/S030438940400648X.
- ↑ Boileau, J.; Wimmer, E.; Gilardi, R.; Stinecipher, M. M.; Gallo, R.; Pierrot, M. (1988-04-15). "Structure of 1,4-dinitroglycoluril". Acta Crystallographica Section C Crystal Structure Communications 44 (4): 696–699. doi:10.1107/S0108270187012204. http://scripts.iucr.org/cgi-bin/paper?S0108270187012204.
 |
