Chemistry:Directing group
In organic chemistry, a directing group (DG) is a substituent on a molecule or ion that facilitates reactions by interacting with a reagent. The term is usually applied to C-H activation of hydrocarbons, where it is defined as a "coordinating moiety (an 'internal ligand'), which directs a metal catalyst into the proximity of a certain C–H bond."[1] In a well known example, the ketone group (C=O) in acetophenone is the DG in the Murai reaction.[1]
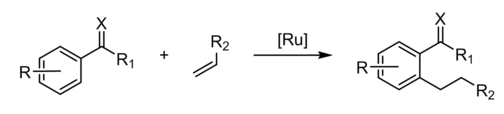
The Murai reaction is related to directed ortho metalation, a reaction is typically applied to the lithiation of substituted aromatic rings.[2]
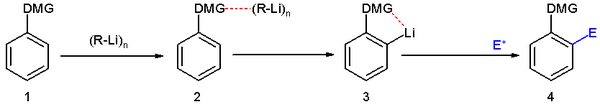
A wide variety of functional groups can serve as directing groups.[3]
Transient directing groups
Since directing groups are ligands, their effectiveness correlates with their affinities for metals. Common functional groups such as ketones usually are only weak ligands and thus often are poor DGs. This problem is solved by the use of a transient directing group. Transient DGs reversibly convert weak DGs (e.g., ketones) into strong DG's (e.g., imines) via a Schiff base condensation. Subsequent to serving their role as DGs, the imine can hydrolyze, regenerating the ketone and amine.[4]

References
- ↑ 1.0 1.1 Sambiagio, C.; D. Schönbauer; R. Blieck; T. Dao-Huy; G. Pototschnig; P. Schaaf; T. Wiesinger; M. F. Zia et al. (2018). "A Comprehensive Overview of Directing Groups Applied in Metal-Catalysed C–H functionalisation chemistry". Chem. Soc. Rev. 47 (17): 6603–6743. doi:10.1039/C8CS00201K. PMID 30033454.
- ↑ Snieckus, Victor (1990). "Directed ortho metalation. Tertiary amide and O-carbamate directors in synthetic strategies for polysubstituted aromatics" Chem. Rev.; 90 (6): 879–933.
- ↑ He, Jian; Wasa, Masayuki; Chan, Kelvin S. L.; Shao, Qian; Yu, Jin-Quan (2017). "Palladium-Catalyzed Transformations of Alkyl C–H Bonds". Chemical Reviews 117 (13): 8754–8786. doi:10.1021/acs.chemrev.6b00622. PMID 28697604.
- ↑ St John-Campbell, S.; J. A. Bull (2018). "Transient imines as 'next generation' directing groups for the catalytic functionalisation of C–H bonds in a single operation". Organic & Biomolecular Chemistry 16 (25): 4582–4595. doi:10.1039/C8OB00926K. PMID 29796566. http://spiral.imperial.ac.uk/bitstream/10044/1/60231/2/MS%20transient%20review%20OBC%20revised.pdf.
 |
