Chemistry:Disodium citrate
From HandWiki
| |
| Names | |
|---|---|
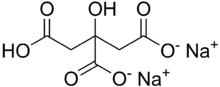
| IUPAC name
Disodium hydrogen 2-hydroxypropane-1,2,3-tricarboxylate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C6H6Na2O7 | |
| Molar mass | 236.087 g·mol−1 |
| Appearance | white crystalline powder |
| Melting point | 149 °C (300 °F; 422 K) |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Disodium citrate, also known as disodium hydrogen citrate, Alkacitron, and sesquihydrate, is an acid salt of citric acid with the chemical formula Na2C6H6O7.[1]
Uses
Food
It is used as an antioxidant in food and to improve the effects of other antioxidants.[2] It is also used as an acidity regulator and sequestrant.[2] Typical products include gelatin, jam, sweets, ice cream, carbonated beverages, milk powder, wine, and processed cheeses. Disodium citrate can also be used as a thickening agent or stabilizer.[3]
Manufacturing
Disodium citrate can also be used as an ingredient in household products that remove stains.[4]
Health
Disodium citrate may be used in patients to alleviate discomfort from urinary-tract infections.[5][6]
References
- ↑ PubChem. "Disodium citrate" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/8950.
- ↑ 2.0 2.1 "Alkarate from Macleods: Disodium Hydrogen Citrate". drugsupdate.com. http://www.drugsupdate.com/brand/generic/Disodium%20Hydrogen%20Citrate/36902.
- ↑ PubChem. "Disodium citrate" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/8950.
- ↑ PubChem. "Disodium citrate" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/8950.
- ↑ "OTC Treatment". https://glowpink.com/blog/cital-syrup/.
- ↑ "Disodium Hydrogen Citrate Syrup". https://labeling.pfizer.com/ShowLabeling.aspx?id=14817.
 |

