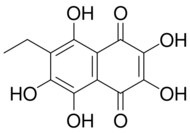
Chemistry:Echinochrome A
| |
| Names | |
|---|---|
| Preferred IUPAC name
6-ethyl-2,3,5,7,8-pentahydroxynaphthalene-1,4-dione | |
| Other names
7-ethyl-2,3,5,6,8-pentahydroxy-1,4-nafphtoquinone; 6-Ethyl-2,7-trihydroxynaphthazarin; 6-ethyl-2,3,5,7,8-pentahydroxy-2-Ethyl-3,5,6,7,8-pentahydroxy-[1,4]naphthoquinone
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| C12H10O7 | |
| Molar mass | 266.20 g/mol |
| Appearance | dark red crystalline powder |
| Melting point | -219 to 221.5°C |
| moderately soluble in ethanol, very slightly soluble in chlorophorm, practically insoluble in water | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Echinochrome A, 7-ethyl-2,3,5,6,8-pentahydroxy-1,4-naphthoquinone is a polyhydroxylated 1,4-naphthoquinone,[1] a type of pigments commonly found in sea urchin shell ("test"), spine, gonads, coelomic fluid, and eggs, of sea urchin.[2] These type of pigments are commonly known as spinochromes and are natural marine phenolic compounds with known and various clinical effects and modes of action.[3]
First extracted from the sea urchin Scaphechinus mirabilis, it is the active substance of histochrome and Echino-A. Histochrome is used for ophthalmic diseases and Ischemic heart disease. Echino-A has been used in nutraceutical form to diminish glucose levels, cholesterol and tryglicerides. The unique properties and the absence of adverse effects of Echinochrome A as a potent antioxidant has made it the focus of intense scientific and clinical studied for more than 30 years.[4][5][6] The several hydroxyl groups have the ability to diminish reactive oxygen species (ROS) in the cells, preventing redox imbalance. Echinochrome A has been found to target ophtalmologic, cardiovascular, cerebrovascular, inflammatory and metabolic diseases through its biological functions by targeting specific molecular signals. The regulation effects produced by echinochrome A in the cells makes this molecule a candidate to improve health.[6][7] Sea urchins are known for their health properties for centuries, for example in the "Materia medica" of the Ming Dynasty authored by Li Zhongli in 1647.[8]
References
- ↑ National Center for Biotechnology Information (2022). PubChem Compound Summary for CID 135457951, Echinochrome A. Retrieved April 13, 2022 from https://pubchem.ncbi.nlm.nih.gov/co
- ↑ Anderson, H.A.; Mathieson, J.W.; Thomson, R.H. (January 1969). "Distribution of spinochrome pigments in echinoids". Comparative Biochemistry and Physiology 28 (1): 333–345. doi:10.1016/0010-406X(69)91347-4. PMID 5777380.
- ↑ Jeong, Seung; Kim, Hyoung; Song, In-Sung; Lee, Seon; Ko, Kyung; Rhee, Byoung; Kim, Nari; Mishchenko, Natalia et al. (13 May 2014). "Echinochrome A Protects Mitochondrial Function in Cardiomyocytes against Cardiotoxic Drugs". Marine Drugs 12 (5): 2922–2936. doi:10.3390/md12052922. PMID 24828295.
- ↑ Hou, Yakun; Vasileva, Elena A.; Carne, Alan; McConnell, Michelle; El-Din A. Bekhit, Alaa; Mishchenko, Natalia P. (2018). "Naphthoquinones of the spinochrome class: occurrence, isolation, biosynthesis and biomedical applications". RSC Advances 8 (57): 32637–32650. doi:10.1039/C8RA04777D. PMID 35547692. Bibcode: 2018RSCAd...832637H.
- ↑ Rubilar, Tamara; Barbieri, Elena S.; Gazquez, Ayelén; Avaro, Marisa (May 2021). "Sea Urchin Pigments: Echinochrome A and Its Potential Implication in the Cytokine Storm Syndrome". Marine Drugs 19 (5): 267. doi:10.3390/md19050267. PMID 34064550.
- ↑ 6.0 6.1 Kim, Hyoung Kyu; Vasileva, Elena A.; Mishchenko, Natalia P.; Fedoreyev, Sergey A.; Han, Jin (August 2021). "Multifaceted Clinical Effects of Echinochrome". Marine Drugs 19 (8): 412. doi:10.3390/md19080412. PMID 34436251.
- ↑ Artyukov, Aleksandr A.; Zelepuga, Elena A.; Bogdanovich, Larisa N.; Lupach, Natalia M.; Novikov, Vyacheslav L.; Rutckova, Tatyana A.; Kozlovskaya, Emma P. (May 2020). "Marine Polyhydroxynaphthoquinone, Echinochrome A: Prevention of Atherosclerotic Inflammation and Probable Molecular Targets". Journal of Clinical Medicine 9 (5): 1494. doi:10.3390/jcm9051494. PMID 32429179.
- ↑ Barbieri, Elena Susana; Rubilar, Tamara; Gázquez, Ayelén; Avaro, Marisa; Seiler, Erina Noé; Vera-Piombo, Mercedes; Gittardi, Agustín; Chaar, Florencia et al. (29 June 2020). Sea Urchin Pigments as Potential Therapeutic Agents Against the Spike Protein of SARS-CoV-2 Based on in Silico Analysis. doi:10.26434/chemrxiv.12568595.v1. This content is a preprint and has not been peer-reviewed
 |

