Chemistry:Edifenphos
From HandWiki
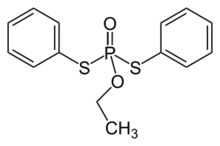
| |
| Names | |
|---|---|
| Preferred IUPAC name
O-Ethyl S,S-diphenyl phosphorodithioate | |
| Other names
O-Ethyl-S,S-diphenyldithiophosphate; EDDP
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C14H15O2PS2 | |
| Molar mass | 310.37 g·mol−1 |
| Density | 1.23 g/cm3[1] |
| Melting point | −25 °C (−13 °F; 248 K)[1] |
| 56 mg/L (20 °C)[1] | |
| Hazards | |
| GHS pictograms |   [1] [1]
|
| GHS Signal word | Danger |
| H301, H311, H331, H317, H410[1] | |
| P261, P273, P280, P301+310, P311, P501[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Edifenphos (O-ethyl-S,S-diphenyldithiophosphate, EDDP) is a systemic fungicide that inhibits phosphatidylcholine biosynthesis.[2][3] It was introduced in 1966 by Bayer to combat blast fungus and Pellicularia sasakii in rice cultivation.[3] It was never authorized for use in the EU.[4]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Record of Edifenphos in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 2016-02-01.
- ↑ Kodama, Osamu; Yamashita, Kenji; Akatsuka, Tadami (1980). "Edifenphos, Inhibitor of Phosphatidylcholine Biosynthesis in Pyricularia oryzae". Agricultural and Biological Chemistry 44 (5): 1015–1021. doi:10.1080/00021369.1980.10864095.
- ↑ 3.0 3.1 Matolcsy, György; Nádasy, Miklós; Andriska, Viktor; Terényi, Sándor (1989). Pesticide Chemistry. Elsevier. p. 306. ISBN 978-0444989031. https://archive.org/details/pesticidechemist00mato.
- ↑ "Edifenphos: Not Approved". EU Pesticides Database Active Substances. http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=activesubstance.selection&language=EN. Retrieved 2016-02-01.
 |
