Chemistry:Eleutheroside D
From HandWiki
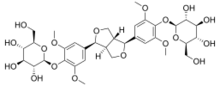
| |
| Names | |
|---|---|
| IUPAC name
(2R,3R,4S,5S,6R)-2-[4-[6-[3,5-dimethoxy-4-[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]-1,3,3a,4,6,6a-hexahydrofuro[3,4-c]furan-3-yl]-2,6-dimethoxyphenoxy]-6-(hydroxymethyl)oxane-3,4,5-triol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C34H46O18 | |
| Molar mass | 742.72 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Eleutheroside D is an eleutheroside.[1] An eleutheroside is a compound found in Eleutherococcus senticosus, the Siberian ginseng. Chemically, it is a dimer of sinapyl alcohol glucoside, and is an optical isomer of Eleutheroside E.[2] Eleutheroside D and E are thought to be the most pharmacologically active out of the eleutherosides.[3]
References
- ↑ Wang, Z.; Zhang, L.; Sun, Y. (2005). "Semipreparative separation and determination of eleutheroside E in Acanthopanax giraldii Harms by high-performance liquid chromatography.". Journal of Chromatographic Science 43 (5): 249–52. doi:10.1093/chromsci/43.5.249. PMID 15975243.
- ↑ Bone, Kerry; Simon Mills, Mcpp (2013). "How to use the monographs". Principles and Practice of Phytotherapy. pp. 353–961. doi:10.1016/B978-0-443-06992-5.00010-4. ISBN 9780443069925.
- ↑ Ovodov, Yu. "Eleutheroside D". https://drugs.ncats.io/drug/WVS8LR64R4.
External links
 |

