Chemistry:Erbium acetylacetonate
From HandWiki
| |
| Identifiers | |
|---|---|
| |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| C15H21ErO6 | |
| Molar mass | 464.586 g·mol−1 |
| Appearance | pink crystals[1] |
| Melting point | 103 °C (376 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
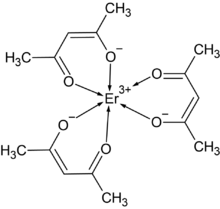
Erbium acetylacetonate is a coordination compound, with the chemical formula of Er(C5H7O2)3, or Er(acac)3 for short. It can be prepared by the reaction of metal erbium[2] or erbium trihydride[1] and acetylacetone. Erbium chloride could also react with ammonium acetylacetonate to obtain erbium acetylacetonate, which can be recrystallized in toluene.[3] Its anhydrous form is stable in dry atmosphere and forms a hydrate in humid air.[4] The anhydrous form cannot be obtained by heating the hydrate in humid atmosphere.[5] It begins to decompose at 190 °C, and erbium oxide can be obtained by continuous heating at 505 °C.[3]
References
- ↑ 1.0 1.1 1.2 Janice M. Koehler, William G. Bos (December 1967). "A novel synthesis of some anhydrous rare earth acetylacetonates" (in en). Inorganic and Nuclear Chemistry Letters 3 (12): 545–548. doi:10.1016/0020-1650(67)80023-0. https://linkinghub.elsevier.com/retrieve/pii/0020165067800230. Retrieved 2021-09-20.
- ↑ J.R. Blackborow, C.R. Eady, E.A.Koerner Von Gustorf, A. Scrivanti, O. Wolfbeis (March 1976). "Chemical syntheses with metal atoms" (in en). Journal of Organometallic Chemistry 108 (3): C32–C34. doi:10.1016/S0022-328X(00)92025-4. https://linkinghub.elsevier.com/retrieve/pii/S0022328X00920254. Retrieved 2021-09-20.
- ↑ 3.0 3.1 J. Blanusa, B. Antic, A. Kremenovic, A.S. Nikolic, L. Mazzerolles, S. Mentus, V. Spasojevic (November 2007). "Particle size effect on Néel temperature in Er2O3 nanopowder synthesized by thermolysis of 2, 4-pentadione complex" (in en). Solid State Communications 144 (7–8): 310–314. doi:10.1016/j.ssc.2007.09.003. Bibcode: 2007SSCom.144..310B. https://linkinghub.elsevier.com/retrieve/pii/S0038109807006448. Retrieved 2021-09-20.
- ↑ Trembovetskii, G. V.; Martynenko, L. I.; Murav'eva, I. A.; Spitsyn (1984). "V. Synthesis and study of volatile rare earth acetylacetonates" (in ru). Doklady Akademii Nauk SSSR 277 (6): 1411–1414. ISSN 0002-3264.
- ↑ Martynenko, L. I.; Murav'eva, I. A.; Khalmurzaev, N. K.; Spitsyn, V. I. Preparation of volatile rare earth element tris(acetylacetonates) by the heating of their hydrates. Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, 1984. 6: 1207-1211. ISSN 0002-3353. language=ru
 |

