Chemistry:Fenobam
| |
| Names | |
|---|---|
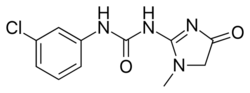
| Preferred IUPAC name
N-(3-Chlorophenyl)-N′-(1-methyl-4-oxo-4,5-dihydro-1H-imidazol-2-yl)urea | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| MeSH | Fenobam |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C11H11ClN4O2 | |
| Molar mass | 266.684 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Fenobam is an imidazole derivative developed by McNeil Laboratories in the late 1970s as a novel anxiolytic drug with an at-the-time-unidentified molecular target in the brain. Subsequently, it was determined that fenobam acts as a potent and selective negative allosteric modulator of the metabotropic glutamate receptor subtype mGluR5,[1][2] and it has been used as a lead compound for the development of a range of newer mGluR5 antagonists.[3][4][5][6]
Fenobam has anxiolytic effects comparable to those of benzodiazepine drugs,[1][7][8] but was never commercially marketed for the treatment of anxiety due to dose-limiting side effects such as amnesia and psychotomimetic symptoms.[9][10] Following the discovery of its activity as a potent negative allosteric modulator of mGluR5, fenobam has been re-investigated for many applications, with its profile of combined antidepressant, anxiolytic, analgesic and anti-addictive effects potentially useful given the common co-morbidity of these symptoms.[11][12] It has also shown promising initial results in the treatment of fragile X syndrome.[13] It was developed by a team at McNeil Laboratories in the 1970s.[14]
Chemistry
Fenobam is known to exist in five crystalline forms, all of them exhibiting a tautomeric structure with the proton attached to the five membered ring nitrogen. [15]
See also
- AZD9272
- Basimglurant
- MPEP
- MTEP
- MFZ 10-7
References
- ↑ 1.0 1.1 Porter RH; Jaeschke G; Spooren W et al. (November 2005). "Fenobam: a clinically validated nonbenzodiazepine anxiolytic is a potent, selective, and noncompetitive mGlu5 receptor antagonist with inverse agonist activity". J. Pharmacol. Exp. Ther. 315 (2): 711–21. doi:10.1124/jpet.105.089839. PMID 16040814.
- ↑ Marino, MJ; Conn, PJ (2006). "Glutamate-based therapeutic approaches: Allosteric modulators of metabotropic glutamate receptors". Current Opinion in Pharmacology 6 (1): 98–102. doi:10.1016/j.coph.2005.09.006. PMID 16368268.
- ↑ Wållberg, A; Nilsson, K; Osterlund, K; Peterson, A; Elg, S; Raboisson, P; Bauer, U; Hammerland, LG et al. (2006). "Phenyl ureas of creatinine as mGluR5 antagonists. A structure-activity relationship study of fenobam analogues". Bioorganic & Medicinal Chemistry Letters 16 (5): 1142–5. doi:10.1016/j.bmcl.2005.11.092. PMID 16380255.
- ↑ Ceccarelli, SM; Jaeschke, G; Buettelmann, B; Huwyler, J; Kolczewski, S; Peters, JU; Prinssen, E; Porter, R et al. (2007). "Rational design, synthesis, and structure-activity relationship of benzoxazolones: New potent mglu5 receptor antagonists based on the fenobam structure". Bioorganic & Medicinal Chemistry Letters 17 (5): 1302–6. doi:10.1016/j.bmcl.2006.12.006. PMID 17189691.
- ↑ Jaeschke, G; Porter, R; Büttelmann, B; Ceccarelli, SM; Guba, W; Kuhn, B; Kolczewski, S; Huwyler, J et al. (2007). "Synthesis and biological evaluation of fenobam analogs as mGlu5 receptor antagonists". Bioorganic & Medicinal Chemistry Letters 17 (5): 1307–11. doi:10.1016/j.bmcl.2006.12.033. PMID 17196387.
- ↑ Gichinga, Moses G.; Olson, Jeremy P.; Butala, Elizabeth; Navarro, Hernán A.; Gilmour, Brian P.; Mascarella, S. Wayne; Carroll, F. Ivy (2011). "Synthesis and Evaluation of Metabotropic Glutamate Receptor Subtype 5 Antagonists Based on Fenobam". ACS Medicinal Chemistry Letters 2 (12): 882–884. doi:10.1021/ml200162f. PMID 22523618.
- ↑ Pecknold, JC; McClure, DJ; Appeltauer, L; Wrzesinski, L; Allan, T (1982). "Treatment of anxiety using fenobam (a nonbenzodiazepine) in a double-blind standard (diazepam) placebo-controlled study". Journal of Clinical Psychopharmacology 2 (2): 129–33. doi:10.1097/00004714-198204000-00010. PMID 7042771.
- ↑ Goldberg, ME; Salama, AI; Patel, JB; Malick, JB (1983). "Novel non-benzodiazepine anxiolytics". Neuropharmacology 22 (12B): 1499–504. doi:10.1016/0028-3908(83)90118-1. PMID 6142427.
- ↑ Palucha, A; Pilc, A (2007). "Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs". Pharmacology & Therapeutics 115 (1): 116–47. doi:10.1016/j.pharmthera.2007.04.007. PMID 17582504.
- ↑ "The anxiolytic and analgesic properties of fenobam, a potent mGlu5 receptor antagonist, in relation to the impairment of learning". Neuropharmacology 57 (2): 97–108. May 2009. doi:10.1016/j.neuropharm.2009.04.011. PMID 19426746.
- ↑ Carroll, FI (2008). "Antagonists at metabotropic glutamate receptor subtype 5: Structure activity relationships and therapeutic potential for addiction". Annals of the New York Academy of Sciences 1141: 221–32. doi:10.1196/annals.1441.015. PMID 18991960.
- ↑ "The mGlu5 antagonist fenobam is analgesic and has improved in vivo selectivity as compared to the prototypical antagonist MPEP". The Journal of Pharmacology and Experimental Therapeutics 330 (3): 834–43. June 2009. doi:10.1124/jpet.109.154138. PMID 19515968.
- ↑ Berry-Kravis, E; Hessl, D; Coffey, S; Hervey, C; Schneider, A; Yuhas, J; Hutchison, J; Snape, M et al. (2009). "A pilot open label, single dose trial of fenobam in adults with fragile X syndrome". Journal of Medical Genetics 46 (4): 266–71. doi:10.1136/jmg.2008.063701. PMID 19126569.
- ↑ US Patent 3983135 4-Oxo-2-imidazolidinylidene ureas
- ↑ Thomas, Sajesh P. (2012). "Polymorphism and tautomeric preference in fenobam and the utility of NLO response to detect polymorphic impurities". Chemical Communications 48 (85): 10559–10561. doi:10.1039/C2CC34912D. PMID 23000909.
 |
