Chemistry:Fluoroethane
From HandWiki
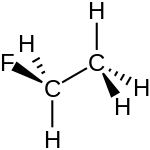
| |
| Names | |
|---|---|
| IUPAC name
Fluoroethane
| |
| Other names
Ethyl fluoride, HFC-161
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 2453 |
| |
| |
| Properties | |
| C2H5F | |
| Molar mass | 48.060 g·mol−1 |
| Appearance | Clear, colourless |
| Odor | Odorless |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H290, H314 | |
| P280, P305+351+338, P310 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LDLo (lowest published)
|
26 pph/4H (rat, inhalation)[1] |
| Related compounds | |
Related compounds
|
Fluoromethane; Fluoropropane; 1,1-Difluoroethane; 1,2-Difluoroethane;1,1,1-Trifluoroethane; 1,1,2-Trifluoroethane; Vinyl fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Fluoroethane (also known as ethyl fluoride) is a hydrofluorocarbon with the chemical formula C
2H
5F). It is a volatile derivative of ethane. It appears as a colourless, odorless flammable gas at room temperature.[3] Fluoroethane can also cause asphyxiation by the displacement of oxygen in air.[4]
Reactivity
Fluoroethane is incompatible with most strong reducing agents and oxidizers. Also, may be incompatible with many amines, nitrides, azo/diazo compounds, with alkali metals, and with epoxides.[5] It is part of the wider class of substances known as fluorinated organic compounds.[6]
See also
References
- ↑ "Fluoroethane". https://pubchem.ncbi.nlm.nih.gov/compound/Fluoroethane#section=Acute-Effects&fullscreen=true.
- ↑ "System of Registries | US EPA". https://sor.epa.gov/sor_internet/registry/substreg/searchandretrieve/substancesearch/search.do?details=displayDetails&selectedSubstanceId=80343#HealthAndOther. Retrieved Sep 26, 2022.
- ↑ PubChem. "Fluoroethane" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/9620.
- ↑ "ETHYL FLUORIDE | CAMEO Chemicals | NOAA". https://cameochemicals.noaa.gov/chemical/3424.
- ↑ PubChem. "Fluoroethane" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/9620.
- ↑ PubChem. "Fluoroethane" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/9620.
 |

