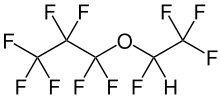
Chemistry:Fluoroether E-1
| |
| Names | |
|---|---|
| Other names
heptafluoropropyl 1,2,2,2-tetrafluoroethyl ether, secondary hydrogen endcap
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C5HF11O | |
| Molar mass | 286.044 g·mol−1 |
| Appearance | Colourless liquid |
| Density | 1.538/cm³ (at 20 °C) |
| Boiling point | 40–42 °C (104–108 °F; 313–315 K) |
| Hazards | |
| Safety data sheet | [1] |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302, H314, H335 | |
| P261, P264, P264+265Script error: No such module "Preview warning".Category:GHS errors, P271, P280, P302+352, P304+340, P305+351+338, P319Script error: No such module "Preview warning".Category:GHS errors, P321, P332+317Script error: No such module "Preview warning".Category:GHS errors, P337+317Script error: No such module "Preview warning".Category:GHS errors, P362+364Script error: No such module "Preview warning".Category:GHS errors, P403+233, P405, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Fluoroether E-1 (known chemically as heptafluoropropyl 1,2,2,2-tetrafluoroethyl ether, is a chemical compound that is among the class of per- and polyfluoroalkyl substances (PFAS). This synthetic fluorochemical is used in the GenX process, and may arise from the degradation of GenX chemicals including FRD-903.[2][3]
Production
The main production of Fluoroether E-1 is within the GenX process where FRD-903 (2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoic acid) is used to generate (FRD-902) ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoate, and Fluoroether E-1 (heptafluoropropyl 1,2,2,2-tetrafluoroethyl ether).[4]
Properties
Fluoroether E-1 is a colorless liquid that is practically insoluble in water. It is volatile and has a low boiling point.[5]
References
- ↑ "Heptafluoropropyl 1,2,2,2-tetrafluoroethyl ether" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/94258#section=Safety-and-Hazards.
- ↑ Zhang, Chuhui; McElroy, Amie C.; Liberatore, Hannah K.; Alexander, Nancy Lee M.; Knappe, Detlef R.U. (2022-05-17). "Stability of Per- and Polyfluoroalkyl Substances in Solvents Relevant to Environmental and Toxicological Analysis". Environmental Science & Technology 56 (10): 6103–6112. doi:10.1021/acs.est.1c03979. ISSN 0013-936X. PMID 34734715.
- ↑ Wickersham, Lindsay (10 March 2023). "Characterization of PFAS air emissions from thermal application of fluoropolymer dispersions on fabrics". Journal of the Air & Waste Management Association 73 (7): 533-552. doi:10.1080/10962247.2023.2192009. https://www.tandfonline.com/doi/full/10.1080/10962247.2023.2192009?src=recsys. Retrieved 11 December 2023.
- ↑ "Antwoord van Gedeputeerde Staten op vragen van W.A. Minderhout (PvdA)" (in Dutch). 2015-09-15. https://www.ozhz.nl/fileadmin/user_upload/2016-02-09_Brief_gedeputeerde_Janssen_en_beantwoording_vragen_BenW_Sliedrecht_over_historische_PFOA-emissies_Chemours_voorheen_Dupont_de_Nemours_bijlagen__nazending_2_.pdf.
- ↑ "Heptafluoropropyl 1,2,2,2-tetrafluoroethyl ether". 2023-10-07. https://store.apolloscientific.co.uk/storage/msds/PC4513_msds.pdf.
 |


