Chemistry:Formyl cyanide
| |
| Names | |
|---|---|
| Preferred IUPAC name
Formyl cyanide | |
| Systematic IUPAC name
Methanoyl cyanide | |
| Other names
Cyanoformaldehyde
Glyoxylonitrile 2-oxo-acetonitrile oxo-acetonitrile | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C2HNO | |
| Molar mass | 55.036 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
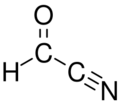
Formyl cyanide is a simple organic compound with the formula HCOCN and structure HC(=O)–C≡N. It is simultaneously a nitrile (R–C≡N) and an aldehyde (R–CH=O). Formyl cyanide is the simplest member of the acyl cyanide family. It is known to occur in space in the Sgr B2 molecular cloud.[1]
Production
Formyl cyanide was first made through methoxyacetonitrile flash vacuum pyrolysis at 600 °C. The same technique with cinnamyloxyacetonitrile[2] or allyloxyacetonitrile also generates formyl cyanide.[3][4]
In molecular clouds, formation of formyl cyanide is speculated to result from formaldehyde and the cyanide radical:[5]
In Earth's atmosphere, the pollutant acrylonitrile reacts with hydroxyl radical forming formyl cyanide, hydroperoxyl and formaldehyde:[6]
Reactions
Formyl cyanide reacts rapidly with even trace quantities of water to form formic acid and hydrogen cyanide. In scrupulously dry conditions, the compound instead releases carbon monoxide, with a half-life exceeding 45 h.[2]
Related
By formally substituting the hydrogen atom, cyanoformyl chloride, ClC(O)CN, and cyanoformyl bromide, BrC(O)CN are obtained.[7]
References
- ↑ Gronowski, Marcin; Eluszkiewicz, Piotr; Custer, Thomas Gage (12 April 2017). "Structure and Spectroscopy of C2HNO Isomers". The Journal of Physical Chemistry A 121 (17): 3263–3273. doi:10.1021/acs.jpca.6b12609. PMID 28402122. Bibcode: 2017JPCA..121.3263G.
- ↑ 2.0 2.1 Lewis-Bevan, Wyn; Gaston, Rick D.; Tyrrell, James; Stork, Wilmer D.; Salmon, Gary L. (March 1992). "Formyl cyanide: a stable species. Experimental and theoretical studies". Journal of the American Chemical Society 114 (6): 1933–1938. doi:10.1021/ja00032a001.
- ↑ Bogey, M.; Destombes, J.L.; Vallee, Y.; Ripoll, J.L. (May 1988). "Formyl cyanide: Efficient production from allyloxyacetonitrile and its millimeter-wave spectrum". Chemical Physics Letters 146 (3–4): 227–229. doi:10.1016/0009-2614(88)87435-9. Bibcode: 1988CPL...146..227B.
- ↑ Bogey, M.; Demuynck, C.; Destombes, J.L.; Vallee, Y. (August 1995). "Millimeter-Wave Spectrum of Formyl Cyanide, HCOCN: Centrifugal Distortion and Hyperfine Structure Analysis". Journal of Molecular Spectroscopy 172 (2): 344–351. doi:10.1006/jmsp.1995.1183. Bibcode: 1995JMoSp.172..344B.
- ↑ Remijan, Anthony J.; Hollis, J. M.; Lovas, F. J.; Stork, Wilmer D.; Jewell, P. R.; Meier, D. S. (10 March 2008). "Detection of Interstellar Cyanoformaldehyde (CNCHO)". The Astrophysical Journal 675 (2): L85–L88. doi:10.1086/533529. Bibcode: 2008ApJ...675L..85R.
- ↑ Grosjean, Daniel (December 1990). "Atmospheric Chemistry of Toxic Contaminants. 3. Unsaturated Aliphatics: Acrolein, Acrylonitrile, Maleic Anhydride". Journal of the Air & Waste Management Association 40 (12): 1664–1669. doi:10.1080/10473289.1990.10466814. Bibcode: 1990JAWMA..40.1664G.
- ↑ Pasinszki, Tibor; Vass, Gábor; Klapstein, Dieter; Westwood, Nicholas P. C. (5 April 2012). "Generation, Spectroscopy, and Structure of Cyanoformyl Chloride and Cyanoformyl Bromide, XC(O)CN". The Journal of Physical Chemistry A 116 (13): 3396–3403. doi:10.1021/jp301528q. PMID 22409314. Bibcode: 2012JPCA..116.3396P.
 |
