Chemistry:Forskolin
| |
| Names | |
|---|---|
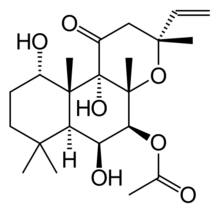
| IUPAC name
(13R)-1α,6β,9α-Trihydroxy-11-oxo-8α,13-epoxylabd-14-en-7β-yl acetate
| |
| Systematic IUPAC name
(3R,4aR,5S,6S,6aS,10S,10aR,10bS)-3-Ethenyl-6,10,10b-trihydroxy-3,4a,7,7,10a-pentamethyl-1-oxododecahydro-1H-naphtho[2,1-b]pyran-5-yl acetate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C22H34O7 | |
| Molar mass | 410.507 g·mol−1 |
| Solubility | Soluble in organic solvents such as ethanol, chloroform and DMSO[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Forskolin (coleonol) is a labdane diterpene produced by the plant Coleus barbatus (blue spur flower). Other names include pashanabhedi, Indian coleus, makandi, HL-362, mao hou qiao rui hua.[2] As with other members of the large diterpene class of plant metabolites, forskolin is derived from geranylgeranyl pyrophosphate (GGPP). Forskolin contains some unique functional elements, including the presence of a tetrahydropyran-derived heterocyclic ring. Forskolin is commonly used in laboratory research to increase levels of cyclic AMP by stimulation of adenylate cyclase.[2]
Mechanism of action
Forskolin is commonly used in biochemistry to raise levels of cyclic AMP (cAMP) in the study and research of cell physiology.[2][3] Forskolin activates the enzyme adenylyl cyclase and increases intracellular levels of cAMP. cAMP is an important second messenger necessary for the proper biological response of cells to hormones and other extracellular signals. It is required for cell communication in the hypothalamus/pituitary gland axis and for the feedback control of hormones via induction of corticotropin-releasing factor gene transcription.[4] Cyclic AMP acts by activating cAMP-sensitive pathways such as protein kinase A and EPAC1.
Chemistry
Derivatives
Its derivatives include colforsin daropate, NKH477,[5] and FSK88,[6] which may be more potent than forskolin at raising cAMP. These derivatives may have pharmaceutical utility against bronchoconstriction and heart failure.[7][8]
Chemical Synthesis
A total chemical synthesis has been reported. The key step of this chemial synthesis is photocyclization of a synthetic intermediate in presence of oxygen and methylene blue, followed by a singlet oxygen Diels-Alder reaction.[9]
Biosynthesis
The heterocyclic ring is synthesized after the formation of the trans-fused carbon ring systems formed by a carbocation mediated cyclization. The remaining tertiary carbocation is quenched by a molecule of water. After deprotonation, the remaining hydroxy group is free to form the heterocyclic ring. This cyclization can occur either by attack of the alcohol oxygen onto the allylic carbocation formed by loss of diphosphate, or by an analogous SN2'-like displacement of the diphosphate.[10] This forms the core ring system A of forskolin.
The remaining modifications of the core ring system A can putatively be understood as a series of oxidation reactions to form a poly-ol B which is then further oxidized and esterified to form the ketone and acetate ester moieties seen in forskolin. However, because the biosynthetic gene cluster has not been described, this putative synthesis could be incorrect in the sequence of oxidation/esterification events, which could occur in almost any order.
Fat loss
In animals, forskolin, or extracts of Coleus barbatus containing forskolin, reduce food intake, body weight, and fat mass. In humans, forskolin reduces body fat mass while increasing lean body mass.[11][12] In mice, extracts of Coleus forskohlii exhibited dose-dependent liver toxicity although purified forskolin did not exhibit liver toxicity.[13]
Other
Forskolin has been used in traditional medicine for treating heart failure.[2]
See also
- Adenylyl cyclase
- Cyclic AMP
References
- ↑ "Forskolin". Sigma Aldrich. http://www.sigmaaldrich.com/etc/medialib/docs/Sigma/Product_Information_Sheet/f6886pis.Par.0001.File.tmp/f6886pis.pdf.
- ↑ 2.0 2.1 2.2 2.3 "Forskolin". Drugs.com. 2018. https://www.drugs.com/npp/forskolin.html.
- ↑ Alasbahi, RH; Melzig, MF (January 2012). "Forskolin and derivatives as tools for studying the role of cAMP.". Die Pharmazie 67 (1): 5–13. doi:10.1691/ph.2012.1642. PMID 22393824.
- ↑ Kageyama, K; Tamasawa, N; Suda, T (July 2011). "Signal transduction in the hypothalamic corticotropin-releasing factor system and its clinical implications.". Stress 14 (4): 357–67. doi:10.3109/10253890.2010.536279. PMID 21438777.
- ↑ "Stimulation of adenylyl cyclase and induction of brain-derived neurotrophic factor and TrkB mRNA by NKH477, a novel and potent forskolin derivative". Journal of Neurochemistry 72 (5): 2198–205. May 1999. doi:10.1046/j.1471-4159.1999.0722198.x. PMID 10217303.
- ↑ "A forskolin derivative, FSK88, induces apoptosis in human gastric cancer BGC823 cells through caspase activation involving regulation of Bcl-2 family gene expression, dissipation of mitochondrial membrane potential and cytochrome c release". Cell Biology International 30 (11): 940–6. November 2006. doi:10.1016/j.cellbi.2006.06.015. PMID 16889987.
- ↑ "Intravenous colforsin daropate, a water-soluble forskolin derivative, prevents thiamylal-fentanyl-induced bronchoconstriction in humans". Critical Care Medicine 30 (4): 820–6. April 2002. doi:10.1097/00003246-200204000-00017. PMID 11940752.
- ↑ "Effects of NKH477, a water-soluble forskolin derivative, on cardiac function in rats with chronic heart failure after myocardial infarction". The Journal of Pharmacology and Experimental Therapeutics 274 (1): 120–6. July 1995. PMID 7616388.
- ↑ "The First Total Synthesis of (±)-Forskolin". Synfacts 18 (5): 0563. May 2022. doi:10.1055/s-0041-1738000.
- ↑ Dewick, P. M. (2009). Medicinal Natural Products (3rd ed.). Wiley. p. 232. ISBN 978-0470741689.
- ↑ Barrea, Luigi; Altieri, Barbara; Polese, Barbara; De Conno, Barbara; Muscogiuri, Giovanna; Colao, Annamaria; Savastano, Silvia (April 2019). "Nutritionist and obesity: brief overview on efficacy, safety, and drug interactions of the main weight-loss dietary supplements". International Journal of Obesity Supplements 9 (1): 32–49. doi:10.1038/s41367-019-0007-3. PMID 31391923.
- ↑ Majeed, Muhammed; Majeed, Shaheen; Nagabhushanam, Kalyanam; Gnanamani, Muthuraman; Mundkur, Lakshmi (February 2021). "Lesser Investigated Natural Ingredients for the Management of Obesity". Nutrients 13 (2): 510. doi:10.3390/nu13020510. PMID 33557185.
- ↑ Jakopin, Žiga (January 2019). "Risks associated with fat burners: A toxicological perspective". Food and Chemical Toxicology 123: 205–224. doi:10.1016/j.fct.2018.10.051. PMID 30401639.
External links
- Hall, Harriet (2014-06-03). "Forskolin: Here We Go Again" (in en-US). Science-Based Medicine. https://sciencebasedmedicine.org/forskolin-here-we-go-again/.
 |
