Chemistry:Furantetracarboxylic acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
Furantetracarboxylic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H4O9 | |
| Molar mass | 244.11 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
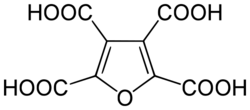
In chemistry, furantetracarboxylic acid is an organic compound with formula C8H4O9, or (C4O)(-(CO)OH)4, which can be viewed as deriving from furan C4H4O through replacement of the four hydrogen atoms by carboxyl functional groups -(CO)OH.
By removal of four protons, the acid is expected to yield the anion C8O4−9, furantetracarboxylate, which is one of the oxocarbon anions (consisting solely of oxygen and carbon. By loss of 1 through 3 protons it forms the anions C8H3O−9, C8H2O2−9, and C8HO3−9, called respectively trihydrogen-, dihydrogen-, and hydrogenfurantetracarboxylate. The same names are used for the corresponding esters.
The acid can be obtained by from dioxalylsuccinate.[1][2][3]
The salt rubidium trihydrogenfurantetracarboxylate RbH3C8O9 crystallizes as white needles.[4]
See also
- Methanetetracarboxylic acid C5H4O8
- Ethylenetetracarboxylic acid C6H4O8
- Benzoquinonetetracarboxylic acid C10H4O10
References
- ↑ B. I. Zapadinskii, B. I. Liogon'kii, and A. A. Berlin (1973), Syntheses of Tetracarboxylic Acids. Russian Chemical Reviews, volume 42 issue 11, page 939. Online version accessed on 2010-01-03.
- ↑ H.Sutter (1932), Annalen, volume 499, page 47. Cited by Zapadinskii et al.
- ↑ T.Reichstein, A.Grussner, K.Schiudlerk, and E. Hardmeyer (1933), Helv.Chim.Acta, volume 16, page 276. Cited by Zapadinskii et al.
- ↑ Iain C. Paul and Leslie L. Martin (1967), The crystal and molecular structure of the monorubidium salt of furantetracarboxylic acid. Acta Crystallogr. volume 22 pages 559-567 doi:10.1107/S0365110X67001136
 |

