Chemistry:GRN-529
From HandWiki
Short description: Chemical compound
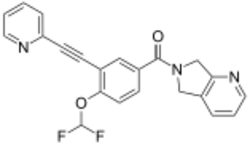 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C22H15F2N3O2 |
| Molar mass | 391.378 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
GRN-529 is a drug that was developed by Wyeth as a negative allosteric modulator of the metabotropic glutamate receptor 5 (mGluR5).[1]
A study conducted by Pfizer found that GRN-529 reduced repetitive behaviors without sedation and partially increased sociability in mouse models of autism. [2]
Another study conducted by Pfizer found a therapeutically relevant effect in animal models of depression. It is theorized to work by reducing glutamate receptor hyperactivity.[3]
See also
References
- ↑ O'Neil SV, Zegarelli BJ, Springer DM, Li DZ, "Bisaryl Alkynylamides as Negative Allosteric Modulators of Metabotropic Glutamate Receptor 5 (MGLUR5)", US patent 2010273772, published 28 October 2010, assigned to Wyeth
- ↑ "Negative allosteric modulation of the mGluR5 receptor reduces repetitive behaviors and rescues social deficits in mouse models of autism". Science Translational Medicine 4 (131): 131ra51. April 2012. doi:10.1126/scitranslmed.3003501. PMID 22539775.
- ↑ "Negative allosteric modulation of metabotropic glutamate receptor 5 results in broad spectrum activity relevant to treatment resistant depression". Neuropharmacology 66: 202–14. March 2013. doi:10.1016/j.neuropharm.2012.04.007. PMID 22551786.
External links
- "GRN-529". Adis Insight. Springer Nature Switzerland AG. http://adisinsight.springer.com/drugs/800033300.
 |

