Chemistry:Germacrene
From HandWiki
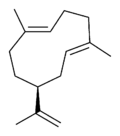
| |
| Names | |
|---|---|
| IUPAC name
(1E,5E,8S)-1,5-dimethyl-8-(prop-1-en-2-yl)cyclodeca-1,5-diene
| |
Other names
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| 6500908 (A) 1864177 (D) | |
| ChEBI |
|
| ChEMBL |
|
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
| C15H24 | |
| Molar mass | 204.35 g/mol |
| Density | 0.793 g/mL |
| Boiling point | 236.4 °C (457.5 °F; 509.5 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
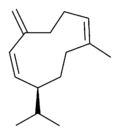
| |
| Names | |
|---|---|
| IUPAC name
(S,1Z,6Z)-8-isopropyl-1-methyl-5-methylidenecyclodeca-1,6-diene
| |
| Other names
1-methyl-5-methylene-8-(1-methylethyl)-1,6-cyclodecadiene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C15H24 | |
| Molar mass | 204.35 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Germacrenes are a class of volatile organic hydrocarbons, specifically, sesquiterpenes. Germacrenes are typically produced in a number of plant species for their antimicrobial and insecticidal properties, though they also play a role as insect pheromones. Two prominent molecules are germacrene A and germacrene D.
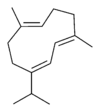
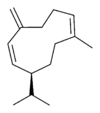
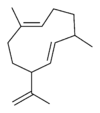
Structures
Germacrene has five isomers.
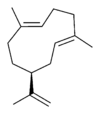
|
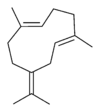
|
|
|
|
| Germacrene A | Germacrene B | Germacrene C | Germacrene D | Germacrene E |
Natural occurrences
The essential oils of red deadnettle (Lamium purpureum)[1] and hedgenettles (genus Stachys)[2] are characterized by their high contents of germacrene D, as is Clausena anisata. It is also a major component of patchouli oil.
References
- ↑ Flamini, G.; Cioni, P. L.; Morelli, I. (2005). "Composition of the essential oils and in vivo emission of volatiles of four Lamium species from Italy: L. purpureum, L. hybridum, L. bifidum and L. amplexicaule". Food Chemistry 91 (1): 63–68. doi:10.1016/j.foodchem.2004.05.047.
- ↑ Morteza‐Semnani, K.; Akbarzadeh, M.; Changizi, Sh. (2005-11-01). "Essential oils composition of Stachys byzantina, S. inflata, S. lavandulifolia and S. laxa from Iran". Flavour and Fragrance Journal 21 (2): 300–303. doi:10.1002/ffj.1594.
Further reading
General
- Adio, A. M. (2009). "Germacrenes A–E and related compounds: thermal, photochemical and acid induced transannular cyclizations". Tetrahedron 65 (8): 1533–1552. doi:10.1016/j.tet.2008.11.050.
Germacrene A
- Deguerry, F.; Pastore, L.; Wu, S.; Clark, A.; Chappell, J.; Schalk, M. (2006-10-15). "The diverse sesquiterpene profile of patchouli, Pogostemon cablin, is correlated with a limited number of sesquiterpene synthases". Archives of Biochemistry and Biophysics 454 (2): 123–136. doi:10.1016/j.abb.2006.08.006. PMID 16970904.
- Omura, H.; Honda, K.; Feeny, P. (2006-09-01). "From terpenoids to aliphatic acids: further evidence for late-instar switch in osmeterial defense as a characteristic trait of swallowtail butterflies in the tribe Papilionini". Journal of Chemical Ecology 32 (9): 1999–2012. doi:10.1007/s10886-006-9124-x. PMID 16902823.
- Forcat, S.; Allemann, R. K. (2006-07-07). "Stabilisation of transition states prior to and following eudesmane cation in aristolochene synthase". Organic and Biomolecular Chemistry 4 (13): 2563–2567. doi:10.1039/b604147g. PMID 16791319.
- Bertea, C. M.; Voster, A.; Verstappen, F. W.; Maffei, M.; Beekwilder, J.; Bouwmeester, H. J. (2006-04-15). "Isoprenoid biosynthesis in Artemisia annua: cloning and heterologous expression of a germacrene A synthase from a glandular trichome cDNA library". Archives of Biochemistry and Biophysics 448 (1–2): 3–12. doi:10.1016/j.abb.2006.02.026. PMID 16579958.
- Lou, Y; Baldwin, I. T. (2006-03-01). "Silencing of a germin-like gene in Nicotiana attenuata improves performance of native herbivores". Plant Physiology 140 (3): 1126–1136. doi:10.1104/pp.105.073700. PMID 16461381.
- Chang, Y.-J.; Jin, J.; Nam, H.-Y.; Kim, S.-U. (2005-03-01). "Point mutation of (+)-germacrene A synthase from Ixeris dentata". Biotechnology Letters 27 (5): 285–288. doi:10.1007/s10529-005-0681-9. PMID 15834787.
Germacrene D
- Rivero Cruz, B.; Rivero Cruz, I; Rodríguez, J. M.; Cerda García-Rojas, C. M.; Mata, R. (2006-08-01). "Qualitative and quantitative analysis of the active components of the essential oil from Brickellia veronicaefolia by nuclear magnetic resonance spectroscopy". Journal of Natural Products '69 (8): 1172–1176. doi:10.1021/np060180b. PMID 16933870.
- Yang, F.-Q.; Li, S.-P.; Chen, Y.; Lao, S.-C.; Wang, Y.-T.; Dong, T.-T.; Tsim, K.-W. (2005-09-15). "Identification and quantitation of eleven sesquiterpenes in three species of Curcuma rhizomes by pressurized liquid extraction and gas chromatography-mass spectrometry". Journal of Pharmaceutical and Biomedical Analysis 39 (3–4): 552–558. doi:10.1016/j.jpba.2005.05.001. PMID 15946818.
- Umlauf, D.; Zapp, J.; Becker, H.; Adam, K. P. (2004-09-01). "Biosynthesis of the irregular monoterpene artemisia ketone, the sesquiterpene germacrene D and other isoprenoids in Tanacetum vulgare L. (Asteraceae)". Phytochemistry 65 (17): 2463–2470. doi:10.1016/j.phytochem.2004.08.019. PMID 15381410. Bibcode: 2004PChem..65.2463U.
- Agnihotri, V. K.; Thappa, R. K.; Meena, B.; Kapahi, B. K.; Saxena, R. K.; Qazi, G. N.; Agarwal, S. G. (2004-08-01). "Essential oil composition of aerial parts of Angelica glauca growing wild in North-West Himalaya (India)". Phytochemistry 65 (16): 2411–2413. doi:10.1016/j.phytochem.2004.07.004. PMID 15381015. Bibcode: 2004PChem..65.2411A.
- Raal, A.; Paaver, U.; Arak, E.; Orav, A. (2004). "Content and composition of the essential oil of Thymus serpyllum L. growing wild in Estonia". Medicina (Kaunas) 40 (8): 795–800. PMID 15300002.
- He, X.; Cane, D. E. (2004-03-10). "Mechanism and stereochemistry of the germacradienol/germacrene D synthase of Streptomyces coelicolor A3(2)". Journal of the American Chemical Society 126 (9): 2678–2679. doi:10.1021/ja039929k. PMID 14995166.
- Arimura, G.-I.; Huber, D. P. W.; Bohlmann, J. (2004). "Forest tent caterpillars (Malacosoma disstria) induce local and systemic diurnal emissions of terpenoid volatiles in hybrid poplar (Populus trichocarpa × deltoides): cDNA cloning, functional characterization, and patterns of gene expression of (−)-germacrene D synthase, PtdTPS1". The Plant Journal 37 (4): 603–616. doi:10.1111/j.1365-313X.2003.01987.x. PMID 14756770.
 |

