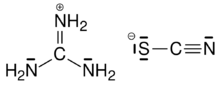
Chemistry:Guanidinium thiocyanate
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C2H6N4S | |
| Molar mass | 118.16 g·mol−1 |
| Hazards | |
| Safety data sheet | External MSDS |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H302, H312, H314, H332, H412 | |
| P260, P261, P264, P270, P271, P273, P280, P301+312, P301+330+331, P302+352, P303+361+353, P304+312, P304+340, P305+351+338, P310, P312, P321, P322, P330, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Guanidinium thiocyanate (GTC) or guanidinium isothiocyanate (GITC) is a chemical compound used as a general protein denaturant, being a chaotropic agent, although it is most commonly used as a nucleic acid protector in the extraction of DNA and RNA from cells.[1]
GITC may also be recognized as guanidine thiocyanate. This is because guanidinium is the conjugate acid of guanidine and is called the guanidinium cation, [CH6N3]+.
Uses
Guanidinium thiocyanate can be used to deactivate a virus, such as the influenza virus that caused the 1918 "Spanish flu", so that it can be studied safely.
Guanidinium thiocyanate is also used to lyse cells and virus particles in RNA and DNA extractions, where its function, in addition to its lysing action, is to prevent activity of RNase enzymes and DNase enzymes by denaturing them. These enzymes would otherwise damage the extract.
A commonly used method is guanidinium thiocyanate-phenol-chloroform extraction. It is not strictly necessary to use phenol or chloroform if extracting RNA for Northern blotting or DNA for Southern blot analysis because the gel electrophoresis followed by transfer to a membrane will separate the RNA/DNA from the proteins. Additionally, since these methods use probes to bind to their conjugates, peptides that get through the process don't generally matter unless a peptide is an RNase or DNase, and then only if the enzyme manages to renature, which should not occur if proper protocols are followed. A possible exception might be when working with temperature extremophiles because some enzymes of these organisms can remain stable under extraordinary circumstances.[2]
Preparation
This substance can be prepared by reacting guanidinium carbonate with ammonium sulfate[3] [4] or ammonium thiocyanate[5] under heat. Another method is the pyrolysis of ammonium thiocyanate[6] or thiourea[7] at 180°C.
See also
- Guanidine hydrochloride
References
- ↑ Mason, P. E.; Neilson, G. W.; Dempsey, C. E.; Barnes, A. C.; Cruickshank, J. M. (2003). "The hydration structure of guanidinium and thiocyanate ions: Implications for protein stability in aqueous solution". Proceedings of the National Academy of Sciences 100 (8): 4557–4561. doi:10.1073/pnas.0735920100. PMID 12684536. Bibcode: 2003PNAS..100.4557M.
- ↑ Shimomura, O; Masugi, T; Johnson, FH; Haneda, Y (March 1978). "Properties and reaction mechanism of the bioluminescence system of the deep-sea shrimp Oplophorus gracilorostris.". Biochemistry 17 (6): 994–98. doi:10.1021/bi00599a008. PMID 629957.
- ↑ Rongfu Li, "Preparation method of guandine thiocyanate", CN patent 103387520, published 2013-11-13
- ↑ Jiawang Wu, "Production method of guanidinium thiocyanate", CN patent 103058889, published 2013-04-24
- ↑ Jianwen Fang, "Prepn of guanidyl thiocyanate", CN patent 1235876, published 2004-09-08
- ↑ Krall, Hans (1913). "CL. – Guanidine thiocyanate: its formation from ammonium thiocyanate". J. Chem. Soc., Trans. 103: 1378–91. doi:10.1039/CT9130301378. https://zenodo.org/record/1517458.
- ↑ Braml, Nicole E.; Sattler, Andreas; Schnick, Wolfgang (6 February 2012). "Formation of Melamium Adducts by Pyrolysis of Thiourea or Melamine/NH4Cl Mixtures". Chemistry – A European Journal 18 (6): 1811–19. doi:10.1002/chem.201101885. PMID 22223531.
 |

