Chemistry:Hexafluorothioacetone
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,1,1,3,3,3-Hexafluoropropane-2-thione | |
| Other names
Perfluorothioacetone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| Properties | |
| C3F6S | |
| Molar mass | 182.08 g·mol−1 |
| Appearance | blue gas |
| Boiling point | 8 °C (46 °F; 281 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
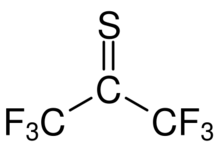
Hexafluorothioacetone is an organic perfluoro thione compound with formula CF3CSCF3. At standard conditions it is a blue gas.[2]
Production
Hexafluorothioacetone was first produced by Middleton in 1961 by boiling bis-(perfluoroisopropyl)mercury with sulfur.
Properties
Hexafluorothioacetone boils at 8 °C.[3] Below this it is a blue liquid.[2]
Colour
The blue colour is due to absorption in the visible light range with bands at 800–675 nm and 725–400 nm. These bands are due to T1–S0 and S1–S0 transitions.[2] There is also a strong absorption in ultraviolet around 230-190 nm.[2]
Reactions
Hexafluorothioacetone acts more like a true thiocarbonyl (C=S) than many other thiocarbonyl compounds, because it is not able to form thioenol compounds (=C-S-H), and the sulfur is not in a negative ionized state (C-S−).[4] Hexafluorothioacetone is not attacked by water or oxygen at standard conditions as are many other thiocarbonyls.[2]
Bases trigger the formation of a dimer 2,2,4,4-tetrakis-(trifluoromethyl)-1,3-dithietane.[2] Bases includes amines.[4]
The dimer can be heated to regenerate the hexafluorothioacetone monomer.[2]
The dimer is also produced in a reaction with hexafluoropropene and sulfur with some potassium fluoride.[2][5]
Hexafluorothioacetone reacts with bisulfite to form a Bunte salt CH(CF3)2SSO2−.[4]
Mercaptans reacting with hexafluorothioacetone yield disulfides or a thiohemiketal:
- R-SH + C(CF3)2S → R-S-S-CH(CF3)2.[4]
- R-SH + C(CF3)2S → RSC(CF3)2SH (for example in methyl mercaptan or ethyl mercaptan).[4]
With mercaptoacetic acid, instead of a thiohemiketal, water elimination yields a ring shaped molecule called a dithiolanone -CH2C(O)SC(CF3)2S- (2,2-di(trifluoromethyl)-1,3-dithiolan-4-one).[4] Aqueous hydrogen chloride results in the formation of a dimeric disulfide CH(CF3)2SSC(CF3)2Cl.[4] Hydrogen bromide with water yields the similar CH(CF3)2SSC(CF3)2Br.[4] Dry hydrogen iodide does something different and reduces the sulfur making CH(CF3)2SH. Wet hydrogen iodide only reduces to a disulfide CH(CF3)2SSC(CF3)2H. Strong organic acids add water to yield a disulfide compound CH(CF3)2SSC(CF3)2OH.[4]
Chlorine and bromine add to hexafluorothioacetone to make CCl(CF3)2SCl and CBr(CF3)2SBr.[4]
With diazomethane hexafluorothioacetone produces 2,2,5,5-tetrakis(trifluoromethyl)-l,3-dithiolane, another substituted dithiolane.[4] Diphenyldiazoniethane reacts to form a three membered ring called a thiirane (di-2,2-trifluoromethyl-di-3,3-phenyl-thiirane)
Trialkylphosphites (P(OR)3) react to make a trialkoxybis(trifluoromethyl)methylenephosphorane (RO)3P=C(CF3)2 and a thiophosphite (RO)3PS.[4]
Hexafluorothioacetone can act as a ligand on nickel.[6]
Hexafluorothioacetone is highly reactive to alkenes and dienes combining via addition reactions. With butadiene it reacts even as low as -78 °C to yield 2,2-bis-(trifluoromethyl)-3,6-dihydro-2H-l-thiapyran.[7]
See also
References
- ↑ Gupta, Kartick; Giri, Santanab; Chattaraj, P. K. (February 2013). "Charge-based DFT descriptors for Diels-Alder reactions". Journal of Physical Organic Chemistry 26 (2): 187–193. doi:10.1002/poc.2987.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Clouthier, Dennis J.; Joo, Duck-Lae (8 May 1997). "The spectroscopy of hexafluorothioacetone, a blue gas". The Journal of Chemical Physics 106 (18): 7479–7490. doi:10.1063/1.473753. Bibcode: 1997JChPh.106.7479C.
- ↑ Knunyants, I. L.; Yakobson, G. G. (2012) (in en). Syntheses of Fluoroorganic Compounds. Springer Science & Business Media. pp. 45–46. ISBN 9783642702075. https://books.google.com/books?id=m6zxCAAAQBAJ&pg=PA45.
- ↑ Jump up to: 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 Middleton, W. J.; Sharkey, W. H. (May 1965). "Fluorothiocarbonyl Compounds. II. Reactions of Hexafluorothioacetone". The Journal of Organic Chemistry 30 (5): 1384–1390. doi:10.1021/jo01016a009.
- ↑ Dyatkin, B.L.; Sterlin, S.R.; Zhuravkova, L.G.; Martynov, B.I.; Mysov, E.I.; Knunyants, I.L. (January 1973). "Reactions of perfluoroalkylcarbanions with sulphur". Tetrahedron 29 (18): 2759–2767. doi:10.1016/S0040-4020(01)93398-8.
- ↑ Browning, Jane; Cundy, C. S.; Green, M.; Stone, F. G. A. (1969). "Hexafluoroacetone and hexafluorothioacetone nickel complexes". Journal of the Chemical Society A: Inorganic, Physical, Theoretical: 20. doi:10.1039/J19690000020.
- ↑ Middleton, W. J.; Howard, E. G.; Sharkey, W. H. (June 1961). "Perfluorothiocarbonyl Compounds". Journal of the American Chemical Society 83 (11): 2589–2590. doi:10.1021/ja01472a045.
External links
 |

