Chemistry:Hopeahainol A
From HandWiki
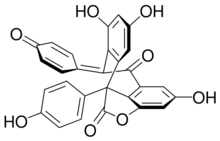
| |
| Names | |
|---|---|
| IUPAC name
(1R,8R,9S,16R)-8,16-Bis(4-hydroxyphenyl)-9-[(1R,8S,9R,16R)-4,6,12-trihydroxy-8,16-bis(4-hydroxyphenyl)-15-oxatetracyclo[8.6.1.02,7.014,17]heptadeca-2(7),3,5,10(17),11,13-hexaen-9-yl]-15-oxatetracyclo[8.6.1.02,7.014,17]heptadeca-2(7),3,5,10(17),11,13-hexaene-4,6,12-triol[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C56H42O12 | |
| Molar mass | 906.940 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hopeahainol A is a polyphenol acetylcholinesterase inhibitor with the molecular formula C56H42O12.[2][3][1][4] Hopeahainol A has been isolated from the tree Hopea hainanensis.[4] Hopeahainol A may be used for the treatment of Alzheimer's disease.[5]
References
- ↑ 1.0 1.1 "Hopeaphenol A" (in en). Pubchem.ncbi.NLM.nih.gov. https://pubchem.ncbi.nlm.nih.gov/compound/Hopeaphenol-A#section=Names-and-Identifiers.
- ↑ Rosenberry, Terrone L.; Martin, Patricia K.; Nix, A. Jeremy; Wildman, Scott A.; Cheung, Jonah; Snyder, Scott A.; Tan, Ren Xiang (November 2016). "Hopeahainol A binds reversibly at the acetylcholinesterase (AChE) peripheral site and inhibits enzyme activity with a novel higher order concentration dependence". Chemico-Biological Interactions 259 (Pt B): 78–84. doi:10.1016/j.cbi.2016.05.032. PMID 27297626.
- ↑ Wiart, Christophe (11 May 2012) (in en). Medicinal Plants of China, Korea, and Japan: Bioresources for Tomorrow's Drugs and Cosmetics. CRC Press. p. 229. ISBN 978-1-4398-9912-0.
- ↑ 4.0 4.1 Ge, Hui Ming; Zhu, Chun Hua; Shi, Da Hua; Zhang, Li Dong; Xie, Dai Qian; Yang, Jie; Ng, Seik Weng; Tan, Ren Xiang (January 2008). "Hopeahainol A: An Acetylcholinesterase Inhibitor from Hopea hainanensis". Chemistry - A European Journal 14 (1): 376–381. doi:10.1002/chem.200700960. PMID 17943703.
- ↑ Zografos, Alexandros L. (18 April 2016) (in en). From Biosynthesis to Total Synthesis: Strategies and Tactics for Natural Products. John Wiley & Sons. p. 370. ISBN 978-1-118-75173-2.
Further reading
- Nicolaou, K. C.; Kang, Qiang; Wu, T. Robert; Lim, Chek Shik; Chen, David Y.-K. (2 June 2010). "Total Synthesis and Biological Evaluation of the Resveratrol-Derived Polyphenol Natural Products Hopeanol and Hopeahainol A". Journal of the American Chemical Society 132 (21): 7540–7548. doi:10.1021/ja102623j. PMID 20462209.
- Chang, Raymond Chuen-Chung (16 December 2011) (in en). Advanced Understanding of Neurodegenerative Diseases. BoD – Books on Demand. p. 170. ISBN 978-953-307-529-7.
 |

