Chemistry:Hydrazine nitrate
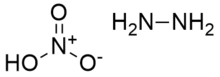
| |
| Names | |
|---|---|
| Other names
hydrazinium nitrate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| Molar mass | 95.02 |
| Appearance | Clear liquid |
| Density | 1.64 g/cm3 |
| Melting point | 72°C |
| Soluble in water | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hydrazine nitrate is an inorganic compound with the chemical formula N
2H
4 · HNO
3. It has usage in liquid explosives as an oxidizer. It exists in two crystalline forms, stable α-type and unstable β-type. The former is usually used in explosives. Its solubility is small in alcohols but
large in water and hydrazine. It has strong hygroscopicity, only slightly lower than ammonium nitrate.[1]
Hydrazine nitrate has a good thermal stability. Its weight loss rate at 100 °C is slower than that of ammonium nitrate. Its explosion point is 307 °C (50% detonation) and explosion heat is about 3.829 MJ/kg. Because it has no carbon elements, the detonation products are not solid and their average molecular weight is small.[1]
Production
Hydrazine nitrate is produced by the reaction of hydrazine and nitric acid:[2]
- N2H4 + HNO3 → N2H5NO3
References
- ↑ 1.0 1.1 Liu, Jiping (2015). Liquid Explosives. Springer. p. 6. doi:10.1007/978-3-662-45847-1. ISBN 9783662458464.
- ↑ D. G. Karraker (1981). Cu(II) - Catalyzed Hydrazine Reduction of Ferric Nitrate (PDF) (Technical report). United States Department of Energy. doi:10.2172/5658572.
Further reading
- Schmidt, Eckart W. (2022). "Hydrazinium Salt Oxidizers". Encyclopedia of Oxidizers. De Gruyter. pp. 1009–1051. doi:10.1515/9783110750294-009. ISBN 978-3-11-075029-4.
 |
