Chemistry:Hydrogen auto-transfer
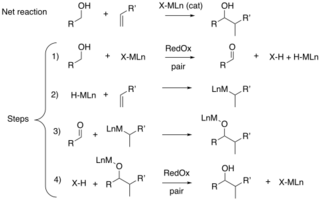
Hydrogen auto-transfer, also known as borrowing hydrogen, is the activation of a chemical reaction by temporary transfer of two hydrogen atoms from the reactant to a catalyst and return of those hydrogen atoms back to a reaction intermediate to form the final product.[1][2][3][4] Two major classes of borrowing hydrogen reactions exist: (a) those that result in hydroxyl substitution,[1][2] and (b) those that result in carbonyl addition.[3][4] In the former case, alcohol dehydrogenation generates a transient carbonyl compound that is subject to condensation followed by the return of hydrogen. In the latter case, alcohol dehydrogenation is followed by reductive generation of a nucleophile, which triggers carbonyl addition. As borrowing hydrogen processes avoid manipulations otherwise required for discrete alcohol oxidation and the use of stoichiometric organometallic reagents, they typically display high levels of atom-economy and, hence, are viewed as examples of Green chemistry.
History
The Guerbet reaction, reported in 1899,[5] is an early example of a hydrogen auto-transfer process. The Guerbet reaction converts primary alcohols to β-alkylated dimers via alcohol dehydrogenation followed by aldol condensation and reduction of the resulting enones. Application of the Guerbet reaction to the development of ethanol-to-butanol processes has garnered interest as a method for the production of renewable fuels.[6] In 1932 using heterogeneous nickel-catalysts Adkins reported the first alcohol aminations that occur through alcohol dehydrogenation-reductive amination.[7] Homogenous catalysts for alcohol amination based on rhodium and ruthenium were developed by Grigg[8] and Watanabe[9] in 1981. The first hydrogen auto-transfer processes that convert primary alcohols to products of carbonyl addition were reported by Michael J. Krische in 2007-2008 using homogenous iridium and ruthenium catalysts.[10][11][12]
Hydroxyl substitution
Alcohol aminations are among the most commonly utilized borrowing hydrogen processes.[13][14][15] In reactions of this type, alcohol dehydrogenation is followed by reductive amination of the resulting carbonyl compound. This represents an alternative to two-step processes involving conversion of the alcohol to a halide or sulfonate ester followed by nucleophilic substitution
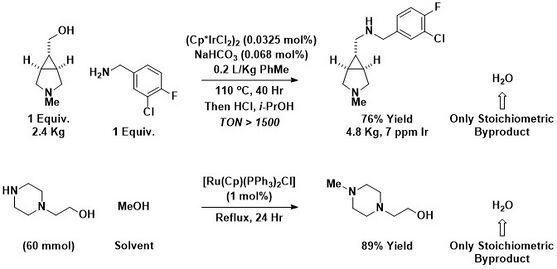
As shown below, alcohol amination has been used on kilogram scale by Pfizer for the synthesis of advanced pharmaceutical intermediates.[16] Additionally, AstraZeneca has used methanol as an alternative to conventional genotoxic methylating agents such as methyl iodide or dimethyl sulfate.[17] Nitroaromatics can also participate as amine precursors in borrowing hydrogen-type alcohol aminations.[18]
The formation of carbon–carbon bond has been achieved through borrowing hydrogen-type Indirect witting,[19] aldol,[20] Knoevenagel condensation [21] and also through various carbon nucleophiles.[22][23] Related to the Guerbet reaction, Donohoe and coworkers have developed enantioselective borrowing hydrogen-type enolate alkylations.[24]
Carbonyl addition
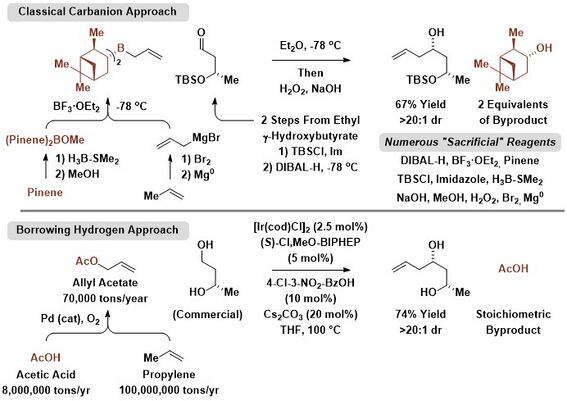
As exemplified by the Krische allylation, dehydrogenation of alcohol reactants can be balanced by reduction of allenes, dienes or allyl acetate to generate allylmetal-carbonyl pairs that combine to give products of carbonyl addition.[3][4] In this way, lower alcohols are directly transformed to higher alcohols in a manner that significantly decreases waste.[25]
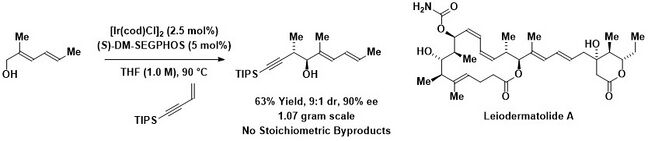
In 2008, borrowing hydrogen reactions of 1,3-enynes with alcohols to form products of carbonyl propargylation was discovered.[26] An enantioselective variant of this method was recently used in the total synthesis of leiodermatolide A.[27]
References
- ↑ 1.0 1.1 1.2 "Borrowing Hydrogen in the Activation of Alcohols" (in en). Advanced Synthesis & Catalysis 349 (10): 1555–1575. 2007. doi:10.1002/adsc.200600638.
- ↑ 2.0 2.1 2.2 "Alcohols as electrophiles in C--C bond-forming reactions: the hydrogen autotransfer process". Angewandte Chemie 46 (14): 2358–64. 2007-03-26. doi:10.1002/anie.200603794. PMID 17465397.
- ↑ 3.0 3.1 3.2 3.3 "Catalytic enantioselective C-H functionalization of alcohols by redox-triggered carbonyl addition: borrowing hydrogen, returning carbon". Angewandte Chemie 53 (35): 9142–50. August 2014. doi:10.1002/anie.201403873. PMID 25056771.
- ↑ 4.0 4.1 4.2 "Metal-catalyzed reductive coupling of olefin-derived nucleophiles: Reinventing carbonyl addition". Science 354 (6310): aah5133. October 2016. doi:10.1126/science.aah5133. PMID 27846504.
- ↑ "Action de l'Alcool Amylique de Fermentation sur Son Dérivé Sodé." (in French). Comptes rendus de l'Académie des Sciences (Paris) 128: 1002–1004. 1899.
- ↑ "Homogeneous Ethanol to Butanol Catalysis—Guerbet Renewed". ACS Catalysis 6 (10): 7125–7132. 2016-10-07. doi:10.1021/acscatal.6b01883. https://orca.cardiff.ac.uk/id/eprint/116206/1/acscatal.6b01883_postprint.pdf.
- ↑ "The alkylation of amines as catalyzed by nickel.". Journal of the American Chemical Society 54 (1): 306–312. 1932-01-01. doi:10.1021/ja01340a046. ISSN 0002-7863.
- ↑ "Transition metal-catalysed N-alkylation of amines by alcohols" (in en). Journal of the Chemical Society, Chemical Communications (12): 611–612. 1981-01-01. doi:10.1039/C39810000611. ISSN 0022-4936.
- ↑ Watanabe, Yoshihisa; Tsuji, Yasushi; Ohsugi, Yukihiro (1981-01-01). "The ruthenium catalyzed N-alkylation and N-heterocyclization of aniline using alcohols and aldehydes" (in en). Tetrahedron Letters 22 (28): 2667–2670. doi:10.1016/S0040-4039(01)92965-X. ISSN 0040-4039.
- ↑ "Catalytic C-C coupling via transfer hydrogenation: reverse prenylation, crotylation, and allylation from the alcohol or aldehyde oxidation level". Journal of the American Chemical Society 129 (49): 15134–5. December 2007. doi:10.1021/ja077389b. PMID 18020342.
- ↑ "Ruthenium-catalyzed C-C bond forming transfer hydrogenation: carbonyl allylation from the alcohol or aldehyde oxidation level employing acyclic 1,3-dienes as surrogates to preformed allyl metal reagents". Journal of the American Chemical Society 130 (20): 6338–9. May 2008. doi:10.1021/ja801213x. PMID 18444617.
- ↑ "Enantioselective iridium-catalyzed carbonyl allylation from the alcohol or aldehyde oxidation level using allyl acetate as an allyl metal surrogate". Journal of the American Chemical Society 130 (20): 6340–1. May 2008. doi:10.1021/ja802001b. PMID 18444616.
- ↑ "The Catalytic Amination of Alcohols" (in en). ChemCatChem 3 (12): 1853–1864. 2011. doi:10.1002/cctc.201100255. ISSN 1867-3899.
- ↑ "Substitution of alcohols by N-nucleophiles via transition metal-catalyzed dehydrogenation". Chemical Society Reviews 44 (8): 2305–29. April 2015. doi:10.1039/C4CS00496E. PMID 25661436.
- ↑ Das, Kuhali; Kumar, Amol; Jana, Akash; Maji, Biplab (2020-03-01). "Synthesis and characterization of N,N-chelate manganese complexes and applications in CN coupling reactions" (in en). Inorganica Chimica Acta 502: 119358. doi:10.1016/j.ica.2019.119358. ISSN 0020-1693. https://www.sciencedirect.com/science/article/pii/S0020169319314793.
- ↑ "Use of an Iridium-Catalyzed Redox-Neutral Alcohol-Amine Coupling on Kilogram Scale for the Synthesis of a GlyT1 Inhibitor". Organic Process Research & Development 15 (5): 1052–1062. 2011-09-16. doi:10.1021/op200174k. ISSN 1083-6160.
- ↑ "A Survey of the Borrowing Hydrogen Approach to the Synthesis of some Pharmaceutically Relevant Intermediates". Organic Process Research & Development 19 (10): 1400–1410. 2015-10-16. doi:10.1021/acs.oprd.5b00199. ISSN 1083-6160. http://eprints.whiterose.ac.uk/95258/3/OPRD%20submission%20%2B%20Revisions%20v2.pdf.
- ↑ "A bifunctional palladium/acid solid catalyst performs the direct synthesis of cyclohexylanilines and dicyclohexylamines from nitrobenzenes". Chemical Communications 49 (74): 8160–2. September 2013. doi:10.1039/c3cc44064h. PMID 23925659.
- ↑ Black, Phillip J.; Edwards, Michael G.; Williams, Jonathan M. J. (2006). "Borrowing Hydrogen: Indirect "Wittig" Olefination for the Formation of C–C Bonds from Alcohols" (in en). European Journal of Organic Chemistry 2006 (19): 4367–4378. doi:10.1002/ejoc.200600070. ISSN 1099-0690. https://onlinelibrary.wiley.com/doi/abs/10.1002/ejoc.200600070.
- ↑ Taguchi, Kazuhiko; Nakagawa, Hideto; Hirabayashi, Tomotaka; Sakaguchi, Satoshi; Ishii, Yasutaka (2004-01-01). "An Efficient Direct α-Alkylation of Ketones with Primary Alcohols Catalyzed by [Ir(cod)Cl2/PPh3/KOH System without Solvent"]. Journal of the American Chemical Society 126 (1): 72–73. doi:10.1021/ja037552c. ISSN 0002-7863. https://doi.org/10.1021/ja037552c.
- ↑ "C–C bond formation from alcohols and malonate half esters using borrowing hydrogen methodology" (in en). Tetrahedron Letters 49 (52): 7413–7415. December 2008. doi:10.1016/j.tetlet.2008.10.059.
- ↑ Blank, Benoît; Kempe, Rhett (2010-01-27). "Catalytic Alkylation of Methyl-N-Heteroaromatics with Alcohols". Journal of the American Chemical Society 132 (3): 924–925. doi:10.1021/ja9095413. ISSN 0002-7863. PMID 20047316. https://doi.org/10.1021/ja9095413.
- ↑ Jana, Akash; Kumar, Amol; Maji, Biplab (2021). "Manganese catalyzed C-alkylation of methyl N -heteroarenes with primary alcohols" (in en). Chemical Communications 57 (24): 3026–3029. doi:10.1039/D1CC00181G. ISSN 1359-7345. PMID 33624678. http://xlink.rsc.org/?DOI=D1CC00181G.
- ↑ "Catalytic Asymmetric Synthesis of Cyclohexanes by Hydrogen Borrowing Annulations". Angewandte Chemie 58 (36): 12558–12562. September 2019. doi:10.1002/anie.201907514. PMID 31265208.
- ↑ "Feedstock Reagents in Metal-Catalyzed Carbonyl Reductive Coupling: Minimizing Preactivation for Efficiency in Target-Oriented Synthesis". Angewandte Chemie 58 (40): 14055–14064. October 2019. doi:10.1002/anie.201905532. PMID 31162793.
- ↑ "Carbonyl propargylation from the alcohol or aldehyde oxidation level employing 1,3-enynes as surrogates to preformed allenylmetal reagents: a ruthenium-catalyzed C-C bond-forming transfer hydrogenation". Angewandte Chemie 47 (28): 5220–3. 2008. doi:10.1002/anie.200801359. PMID 18528831.
- ↑ "Total Synthesis of Leiodermatolide A via Transfer Hydrogenative Allylation, Crotylation, and Propargylation: Polyketide Construction beyond Discrete Allyl- or Allenylmetal Reagents". Journal of the American Chemical Society 143 (28): 10590–10595. July 2021. doi:10.1021/jacs.1c06062. PMID 34237219.
 |