Chemistry:Hydrohalite
| Hydrohalite | |
|---|---|
| General | |
| Category | Halide mineral |
| Formula (repeating unit) | NaCl · 2H2O |
| Strunz classification | 3.BA.05 |
| Dana classification | 9.1.2.1 |
| Crystal system | Monoclinic |
| Crystal class | Prismatic (2/m) (same H-M symbol) |
| Space group | P21/c |
| Identification | |
| Colour | Colourless or white |
| Diaphaneity | Transparent |
Hydrohalite is a halide mineral that occurs in saturated halite brines at cold temperatures (below 0.1 °C) and is the most common form of hydrated sodium chloride. It was first described in 1847 from an occurrence in Dürrnberg, Austria.
Physical properties
Hydrohalite has a high nucleation energy, it decomposes at 0.1°C, giving a salty brine and solid halite.
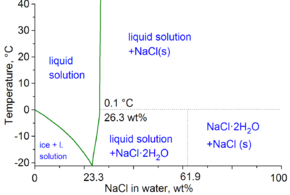
The cryohydric point of hydrohalite is at −21.2 °C (−6.2 °F), solutions will normally need to be supercooled for crystals to form. Above this temperature, liquid water saturated with salt can exist in equilibrium with hydrohalite. Unlike halite, hydrohalite has a strong positive temperature coefficient of solubility.[2] Under pressure, hydrohalite is stable between 7,900 and 11,600 atmospheres pressure. The decomposition point increases at the rate of 0.007K per atmosphere (for 1–1000 atmospheres),[2] reaching a maximum decomposition temperature is at 25.8°C around 9400 atmospheres. The decomposition temperature reduces again at higher pressures.[2]
Occurrence
The type locality is the Hallein Salt Mine in Austria.[3]
Ceres
Hydrohalite was discovered on Ceres by Dawn,[4] suggesting an early ocean, possibly surviving as a relict ocean.
References
- ↑ Warr, L.N. (2021). "IMA–CNMNC approved mineral symbols". Mineralogical Magazine 85 (3): 291–320. doi:10.1180/mgm.2021.43. Bibcode: 2021MinM...85..291W.
- ↑ 2.0 2.1 2.2 Braitsch, O. (1971). "The Stability Conditions of Salt Minerals". Salt Deposits Their Origin and Composition. Springer. pp. 42–44. doi:10.1007/978-3-642-65083-3_2. ISBN 978-3-642-65085-7.
- ↑ Page Hydrohalite: Mineral information, data and localities on "mindat.org". Hudson Institute of Mineralogy. https://www.mindat.org/min-1975.html.
- ↑ De Sanctis, M.C., Ammannito, E., Raponi, A. et al. Fresh emplacement of hydrated sodium chloride on Ceres from ascending salty fluids. Nat Astron 4, 786–793 (2020). https://doi.org/10.1038/s41550-020-1138-8
 |
