Chemistry:Hydroiodic acid
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Iodane[1]
| |||
| Other names
Hydronium iodide
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| |||
| |||
| Properties | |||
| HI(aq) | |||
| Molar mass | 127.91 g/mol | ||
| Appearance | colorless liquid | ||
| Odor | acrid | ||
| Density | 1.70 g/mL, azeotrope (57% HI by weight) | ||
| Boiling point | 127 °C (261 °F; 400 K) 1.03 bar, azeotrope | ||
| Aqueous solution | |||
| Acidity (pKa) | -9.3 | ||
| Hazards | |||
| GHS pictograms | 
| ||
| GHS Signal word | Danger | ||
| H314 | |||
| P260, P264, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Related compounds | |||
Other anions
|
Hydrofluoric acid Hydrochloric acid Hydrobromic acid | ||
Related compounds
|
Hydrogen iodide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Hydroiodic acid (or hydriodic acid) is a colorless and aqueous solution of hydrogen iodide (HI). It is a strong acid, which is ionized completely in an aqueous solution. Concentrated solutions of hydroiodic acid are usually 48% to 57% HI.[2]

Reactions
Hydroiodic acid reacts with oxygen in air to give iodine:
- 4 HI + O2 → 2 H2O + 2 I2
Like other hydrogen halides, hydroiodic acid adds to alkenes to give alkyl iodides. It can also be used as a reducing agent, for example in the reduction of aromatic nitro compounds to anilines.[3]
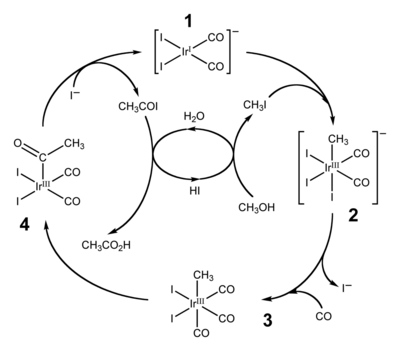
Cativa process
The Cativa process is a major end use of hydroiodic acid, which serves as a co-catalyst for the production of acetic acid by the carbonylation of methanol.[4][5]
Illicit uses
Hydroiodic acid is listed as a U.S. Federal DEA List I Chemical, owing to its use as a reducing agent related to the production of methamphetamine from ephedrine or pseudoephedrine (recovered from nasal decongestant pills).[6]
References
- ↑ Henri A. Favre, ed (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. Cambridge: The Royal Society of Chemistry. p. 131.
- ↑ Lyday, Phyllis A. (2005). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. pp. 382–390. doi:10.1002/14356007.a14_381.
- ↑ Kumar, J. S. Dileep; Ho, ManKit M.; Toyokuni, Tatsushi (2001). "Simple and chemoselective reduction of aromatic nitro compounds to aromatic amines: reduction with hydriodic acid revisited". Tetrahedron Letters 42 (33): 5601–5603. doi:10.1016/s0040-4039(01)01083-8.
- ↑ Jones, J. H. (2000). "The Cativa Process for the Manufacture of Acetic Acid". Platinum Metals Rev. 44 (3): 94–105. http://www.platinummetalsreview.com/pdf/pmr-v44-i3-094-105.pdf.
- ↑ Sunley, G. J.; Watson, D. J. (2000). "High productivity methanol carbonylation catalysis using iridium - The Cativa process for the manufacture of acetic acid". Catalysis Today 58 (4): 293–307. doi:10.1016/S0920-5861(00)00263-7.
- ↑ Skinner, Harry F. (1990). "Methamphetamine synthesis via hydriodic acid/Red phosphorus reduction of ephedrine". Forensic Science International 48 (2): 123–134. doi:10.1016/0379-0738(90)90104-7.
External links
- International Chemical Safety Card 1326
- European Chemicals Bureau
- Viscosities of Aqueous Hydrochloric Acid Solutions, and Densities and Viscosities of Aqueous Hydroiodic Acid Solutions
nl:Waterstofjodide pl:Kwas jodowodorowy
 |