Chemistry:Hydroxamic acid
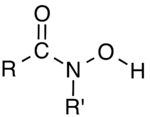
In organic chemistry, hydroxamic acids are a class of organic compounds bearing the functional group R–C(=O)–N(OH)–R', with R and R' as organic residues. They are amides (R–C(=O)–NH–R') wherein the nitrogen center has a hydroxyl (–OH) substituent. They are often used as metal chelators.[1]
Synthesis and reactions
Hydroxamic acids are usually prepared from either esters or acid chlorides by a reaction with hydroxylamine salts. For the synthesis of benzohydroxamic acid, the overall equation is:[2]
- C6H5CO2Me + NH2OH → C6H5C(O)NHOH + MeOH
Hydroxamic acids can also be synthesized from aldehydes and N-sulfonylhydroxylamine via the Angeli-Rimini reaction.[3]
A well-known reaction of hydroxamic acid esters is the Lossen rearrangement.[4]
Coordination chemistry and biochemistry
- Sample gallery
-
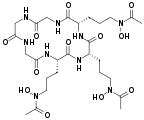
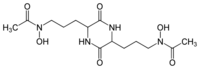
Fe(III) complex of triacetylfusarinine[5]
The conjugate base of hydroxamic acids forms is called a hydroxamate. Deprotonation occurs at the NOH group. The resulting conjugate base presents the metal with an anionic, conjugated O,O chelating ligand. Many hydroxamic acids and many iron hydroxamates have been isolated from natural sources.[6]
They function as ligands, usually for iron.[7] Nature has evolved families of hydroxamic acids to function as iron-binding compounds (siderophores) in bacteria. They extract iron(III) from otherwise insoluble sources (rust, minerals, etc.). The resulting complexes are transported into the cell, where the iron is extracted and utilized metabolically.[8]
Ligands derived from hydroxamic acid and thiohydroxamic acid also form strong complexes with lead(II).[9]
Other uses and occurrences
Hydroxamic acids are used extensively in flotation of rare earth minerals during the concentration and extraction of ores to be subjected to further processing.[10][11]
Some hydroxamic acids (e.g. vorinostat, belinostat, panobinostat, and trichostatin A) are HDAC inhibitors with anti-cancer properties. Fosmidomycin is a natural hydroxamic acid inhibitor of 1-deoxy-D-xylulose-5-phosphate reductoisomerase (DXP reductoisomerase). Hydroxamic acids have also been investigated for reprocessing of irradiated fuel.[citation needed]
References
- ↑ Munson, James W. (1992). "Hydroxamic acids". in S. Patai. Acid Derivatives (1992), Volume 2. PATAI'S Chemistry of Functional Groups. pp. 849–873. doi:10.1002/9780470772508.ch15. ISBN 9780470772508.
- ↑ C. R. Hauser; W. B. Renfrow Jr (1939). "Benzohydroxamic Acid". Org. Synth. 19: 15. doi:10.15227/orgsyn.019.0015.
- ↑ Li, Jie Jack (2003). Name Reactions: A Collection of Detailed Reaction Mechanisms (2nd ed.). Berlin, Heidelberg, New York: Springer. p. 9. ISBN 978-3-662-05338-6.
- ↑ Wang, Zerong (2010). Comprehensive organic name reactions and reagents. John Wiley & Sons, Inc.. pp. 1772–1776. ISBN 9780471704508.
- ↑ Hossain, M. B.; Eng-Wilmot, D. L.; Loghry, R. A.; an der Helm, D. (1980). "Circular Dichroism, Crystal Structure, and Absolute Configuration of the Siderophore Ferric N,N',N"-Triacetylfusarinine, FeC39H57N6O15". Journal of the American Chemical Society 102 (18): 5766–5773. doi:10.1021/ja00538a012.
- ↑ Abraham Shanzer, Clifford E. Felder, Yaniv Barda (2008). "Natural and Biomimetic Hydroxamic Acid based Siderophores". in Zvi Rappoport, Joel F. Liebman. The Chemistry of Hydroxylamines, Oximes and Hydroxamic Acids. PATAI'S Chemistry of Functional Groups. pp. 751–815. doi:10.1002/9780470741962.ch16. ISBN 9780470512616.
- ↑ Agrawal, Y K (1979). "Hydroxamic Acids and Their Metal Complexes". Russian Chemical Reviews 48 (10): 948–963. doi:10.1070/RC1979v048n10ABEH002422. Bibcode: 1979RuCRv..48..948A.
- ↑ Miller, Marvin J. (November 1989). "Syntheses and Therapeutic Potential of Hydroxamic Acid Based Siderophores and Analogues". Chemical Reviews 89 (7): 1563–1579. doi:10.1021/cr00097a011.
- ↑ Farkas, Etelka; Buglyó, Péter (2017). "Chapter 8. Lead(II) Complexes of Amino Acids, Peptides, and Other Related Ligands of Biological Interest". in Astrid, S.; Helmut, S.; Sigel, R. K. O.. Lead: Its Effects on Environment and Health. Metal Ions in Life Sciences. 17. de Gruyter. pp. 201–240. doi:10.1515/9783110434330-008. ISBN 9783110434330.
- ↑ Marion, Christopher; Jordens, Adam; Li, Ronghao; Rudolph, Martin; Waters, Kristian E. (August 2017). "An evaluation of hydroxamate collectors for malachite flotation". Separation and Purification Technology 183: 258–269. doi:10.1016/j.seppur.2017.02.056.
- ↑ Jordens, Adam; Cheng, Ying Ping; Waters, Kristian E. (February 2013). "A review of the beneficiation of rare earth element bearing minerals". Minerals Engineering 41: 97–114. doi:10.1016/j.mineng.2012.10.017. Bibcode: 2013MiEng..41...97J.
Further reading
- Fouché, K. F.; H. J. le Roux; F. Phillips (June 1970). "Complex formation of Zr(IV) and Hf(IV) with hydroxamic acids in acidic solutions". Journal of Inorganic and Nuclear Chemistry 32 (6): 1949–1962. doi:10.1016/0022-1902(70)80604-2. ISSN 0022-1902. http://www.science-direct.com/science/article/B758S-48M3JS8-NH/2/906cec1e859eacb47bf1f0efcd27e5cc. Retrieved 2009-04-24.
- Barocas, A.; F. Baroncelli; G. B. Biondi; G. Grossi (December 1966). "The complexing power of hydroxamic acids and its effect on behaviour of organic extractants in the reprocessing of irradiated fuels--II : The complexes between benzohydroxamic acid and thorium, uranium (IV) and plutonium (IV)". Journal of Inorganic and Nuclear Chemistry 28 (12): 2961–2967. doi:10.1016/0022-1902(66)80023-4. ISSN 0022-1902. http://www.science-direct.com/science/article/B758S-48GWMMM-8P/2/0e24f83e069f7473d7b0663eea4f8302. Retrieved 2009-04-24.
- Baroncelli, F.; G. Grossi (May 1965). "The complexing power of hydroxamic acids and its effect on the behaviour of organic extractants in the reprocessing of irradiated fuels--I the complexes between benzohydroxamic acid and zirconium, iron (III) and uranium (VI)". Journal of Inorganic and Nuclear Chemistry 27 (5): 1085–1092. doi:10.1016/0022-1902(65)80420-1. ISSN 0022-1902. http://www.science-direct.com/science/article/B758S-48N50RN-2W/2/fc4a67c10211cd748add5daa51bb55ff. Retrieved 2009-04-24.
- Al-Jarrah, R. H.; A. R. Al-Karaghouli; S. A. Al-Assaf; N. H. Shamon (1981). "Solvent extraction of uranium and some other metal ions with 2-N-butyl-2-ethyl octanohydroxamic acid". Journal of Inorganic and Nuclear Chemistry 43 (11): 2971–2973. doi:10.1016/0022-1902(81)80652-5. ISSN 0022-1902. http://www.science-direct.com/science/article/B758S-48M3SCR-152/2/723dc6c17d83f6266314116734ae71e8. Retrieved 2009-04-24.
- Gopalan, Aravamudan S.; Vincent J. Huber; Orhan Zincircioglu; Paul H. Smith (1992). "Novel tetrahydroxamate chelators for actinide complexation: synthesis and binding studies". Journal of the Chemical Society, Chemical Communications (17): 1266–1268. doi:10.1039/C39920001266. https://zenodo.org/record/1230022.
- Koshti, Nirmal; Vincent Huber; Paul Smith; Aravamudan S. Gopalan (1994-02-28). "Design and synthesis of actinide specific chelators: Synthesis of new cyclam tetrahydroxamate (CYTROX) and cyclam tetraacetonylacetone (CYTAC) chelators". Tetrahedron 50 (9): 2657–2664. doi:10.1016/S0040-4020(01)86981-7. ISSN 0040-4020. https://zenodo.org/record/1259713.
 |
![Fe(III) complex of triacetylfusarinine[5]](/wiki/images/thumb/5/5b/Fe%28hydroxamate%293.svg/125px-Fe%28hydroxamate%293.svg.png)
