Chemistry:Lossen rearrangement
| Lossen rearrangement | |
|---|---|
| Named after | Wilhelm Lossen |
| Reaction type | Rearrangement reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000156 |
The Lossen rearrangement is the conversion of a hydroxamate ester to an isocyanate. Typically O-acyl, sulfonyl, or phosphoryl O-derivative are employed.[1][2][3][4] The isocyanate can be used further to generate ureas in the presence of amines or generate amines in the presence of H2O.
Reaction mechanism
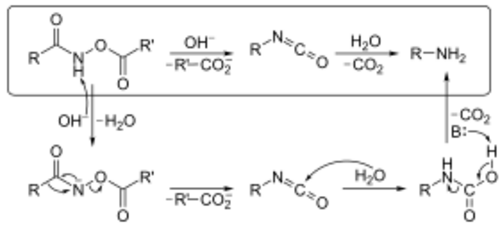
The mechanism below begins with an O-acylated hydroxamic acid derivative that is treated with base to form an isocyanate that generates an amine and CO2 gas in the presence of H2O. The hydroxamic acid derivative is first converted to its conjugate base by abstraction of a hydrogen by a base. Spontaneous rearrangement releases a carboxylate anion to produce the isocyanate intermediate. The isocyanate is then hydrolyzed in the presence of H2O. Finally, the respective amine and CO2 are generated by abstraction of a proton with a base and decarboxylation.
Hydroxamic acids are commonly synthesized from their corresponding esters.[5]
Historical references
- Lossen, W. (1872). "Ueber Benzoylderivate des Hydroxylamins". Justus Liebigs Annalen der Chemie 161 (2–3): 347–362. doi:10.1002/jlac.18721610219. https://zenodo.org/record/1427305.
- Lossen, W. (1875). "Ueber die Structurformel des Hydroxylamins und seiner amidartigen Derivate". Justus Liebigs Annalen der Chemie 175 (3): 271–304. doi:10.1002/jlac.18751750303. https://zenodo.org/record/1427343.
- Lossen, W. (1875). "Methode, die Carboxylgruppe aromatischer Säuren durch die Amidgruppe zu ersetzen". Justus Liebigs Annalen der Chemie 175 (3): 313–325. doi:10.1002/jlac.18751750305. https://zenodo.org/record/1427345.
See also
References
- ↑ Wang, Zerong (2010). Comprehensive organic name reactions and reagents. John Wiley & Sons, Inc.. pp. 1772–1776. ISBN 9780471704508.
- ↑ Yale, H. L. (1943). "The Hydroxamic Acids". Chem. Rev. 33 (3): 209–256. doi:10.1021/cr60106a002.
- ↑ Bauer, L.; Exner, O. (1974). "The Chemistry of Hydroxamic Acids and N-Hydroxyimides". Angew. Chem. Int. Ed. Engl. 13 (6): 376. doi:10.1002/anie.197403761.
- ↑ Shioiri, Takayuki (1991). "Degradation Reactions". Comprehensive Organic Synthesis 6: 795–828. doi:10.1016/B978-0-08-052349-1.00172-4. ISBN 9780080359298.
- ↑ Hauser, C. R.; Renfrow, Jr.., W. B. (1939). "Benzohydroxamic Acid". Organic Syntheses 19: 15. doi:10.15227/orgsyn.019.0015.
External links
 |