Chemistry:Hydroxy-1,4-benzoquinone
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Hydroxycyclohexa-2,5-diene-1,4-dione | |||
| Other names
2-Hydroxy-p-benzoquinone
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C6H4O3 | |||
| Molar mass | 124.1 g/mol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
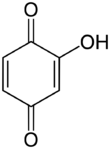
Hydroxy-1,4-benzoquinone, also called hydroxy-para-benzoquinone, is an organic compound with formula C6H4O3, formally derived from 1,4-Benzoquinone by replacing one hydrogen atom with a hydroxyl (OH) group. It is one of three hydroxybenzoquinone isomers and one of the simplest hydroxyquinones.
The compound is often called 2-hydroxy-1,4-benzoquinone, but the "2-" prefix is superfluous since there is no other hydroxy derivative of 1,4-benzoquinone. The IUPAC name is 2-hydroxycyclohexa-2,5-diene-1,4-dione.
It is formed by the reaction of 1,4-benzoquinone with hydrogen peroxide and is a byproduct of the metabolism of phenols, such as 1,2,4-benzenetriol.[1] The enzyme 1,2,4-benzenetriol dehydrogenase catalyzes the conversion of 1,2,4-benzenetriol to 2-hydroxy-1,4-benzoquinone, and the enzyme hydroxybenzoquinone reductase catalyzes the reverse reaction. The enzyme 2-hydroxy-1,4-benzoquinone-2-reductase converts it to 1,4-benzoquinone.
It tends to dimerize spontaneously by peroxo bridges.[1]
See also
References
- ↑ 1.0 1.1 Philipp, B; Schink, B (1998). "Evidence of two oxidative reaction steps initiating anaerobic degradation of resorcinol (1,3-dihydroxybenzene) by the denitrifying bacterium Azoarcus anaerobius". Journal of Bacteriology 180 (14): 3644–9. PMID 9658009.
 |