Chemistry:Hydroxybutyric acid
From HandWiki
Skeletal formula of α-hydroxybutyric acid
Hydroxybutyric acid is a group of four-carbon organic compounds that have both hydroxyl and carboxylic acid functional groups.[1] They can be viewed as derivatives of butyric acid. The carboxylate anion and the esters of hydroxybutyric acids are known as hydroxybutyrates. β-hydroxybutyric acid is relevant to human health as it is a member of a class of products of fatty acid oxidation referred to as ketone bodies.
The isomers are distinguished by the distance between the two functional groups and the branching.
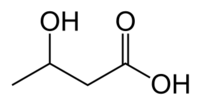
- alpha-Hydroxybutyric acid (2-hydroxybutyric acid)
- beta-Hydroxybutyric acid (3-hydroxybutyric acid)
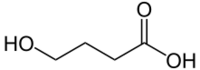
- gamma-Hydroxybutyric acid (4-hydroxybutyric acid, GHB)
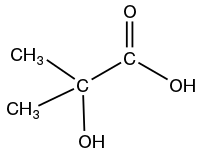
- 2-hydroxyisobutyric acid
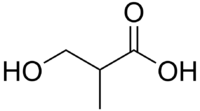
- 3-hydroxyisobutyric acid
See also
References
- ↑ Miltenberger, Karlheinz (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_507.
External links
- Hydroxybutyrates at the US National Library of Medicine Medical Subject Headings (MeSH)
 |