Chemistry:2-Hydroxyisobutyric acid
| |
| Names | |
|---|---|
| Other names
α-Hydroxyisobutyric acid
2-Hydroxyisobutanoic acid α-Hydroxyisobutanoic acid 2-Hydroxy-2-methyl propanoic acid 2-Hydroxy-2-methyl propionic acid 2-Methyllactic acid 2-Methylglycolic acid Acetonic acid Hydroxydimethylacetic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| 1744739 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C4H8O3 | |
| Molar mass | 104.105 g·mol−1 |
| Appearance | white solid |
| Density | 1.23 g/cm3[1] |
| Melting point | 82.5 °C (180.5 °F; 355.6 K) |
| Boiling point | 212 °C (414 °F; 485 K) |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H302, H315, H318, H335 | |
| P261, P264, P264+265Script error: No such module "Preview warning".Category:GHS errors, P270, P271, P280, P301+317Script error: No such module "Preview warning".Category:GHS errors, P302+352, P304+340, P305+354+338Script error: No such module "Preview warning".Category:GHS errors, P317Script error: No such module "Preview warning".Category:GHS errors, P319Script error: No such module "Preview warning".Category:GHS errors, P321, P330, P332+317Script error: No such module "Preview warning".Category:GHS errors, P362+364Script error: No such module "Preview warning".Category:GHS errors, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
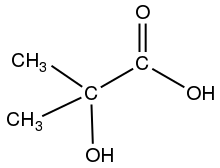
2-Hydroxyisobutyric acid is the organic compound with the formula (CH
3)
2C(OH)CO
2H. A white solid, it is classified as an hydroxycarboxylic acid. It has been considered as a naturally occurring precursor to polyesters.[3] It is closely related to lactic acid (CH
3CH(OH)CO
2H).[4]
Occurrences
The enzyme 2-hydroxyisobutyryl coenzyme A (CoA) mutase isomerizes 3-hydroxybutyryl coenzyme A into 2-hydroxyisobutyryl coenzyme A. Hydrolysis of the latter gives 2-hydroxyisobutyric acid.[5]
The amides formed from this carboxylic acid and the ε-amino group of lysine residues are a kind of post translational modification.[6][7]
Ethyl methacrylate (an industrially important monomer and ester of methacrylic acid) was first obtained by treating the ethyl ester of 2-hydroxyisobutyric acid with phosphorus pentachloride in an apparent dehydration reaction.[8]
See also
References
- ↑ W.P.J.Gaykema; J.A.Kanters; G.Roelofsen (1978), Cryst. Struct. Commun., 47: 463
- ↑ "2-Hydroxyisobutyric acid" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/11671#section=Safety-and-Hazards.
- ↑ Bhatia, Shashi Kant; Bhatia, Ravi Kant; Yang, Yung-Hun (2016). "Biosynthesis of Polyesters and Polyamide Building Blocks Using Microbial Fermentation and Biotransformation". Reviews in Environmental Science and Bio/Technology 15 (4): 639–663. doi:10.1007/s11157-016-9415-9.
- ↑ Miltenberger, Karlheinz (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_507.
- ↑ Rohde, Maria-Teresa; Tischer, Sylvi; Harms, Hauke; Rohwerder, Thore (2017). "Production of 2-Hydroxyisobutyric Acid from Methanol by Methylobacterium extorquens AM1 Expressing ( R )-3-Hydroxybutyryl Coenzyme A-Isomerizing Enzymes". Applied and Environmental Microbiology 83 (3). doi:10.1128/AEM.02622-16. PMID 27836853. Bibcode: 2017ApEnM..83E2622R.
- ↑ Dai, Lunzhi; Peng, Chao; Montellier, Emilie; Lu, Zhike; Chen, Yue; Ishii, Haruhiko; Debernardi, Alexandra; Buchou, Thierry et al. (2014). "Lysine 2-Hydroxyisobutyrylation is a Widely Distributed Active Histone Mark". Nature Chemical Biology 10 (5): 365–370. doi:10.1038/nchembio.1497. PMID 24681537.
- ↑ Carrola, Joana; Rocha, CláUdia M.; Barros, António S.; Gil, Ana M.; Goodfellow, Brian J.; Carreira, Isabel M.; Bernardo, João; Gomes, Ana et al. (2011). "Metabolic Signatures of Lung Cancer in Biofluids: NMR-Based Metabonomics of Urine". Journal of Proteome Research 10 (1): 221–230. doi:10.1021/pr100899x. PMID 21058631.
- ↑ E. Frankland, B. F. Duppa (1865). "Untersuchungen über Säuren aus der Acrylsäure-Reihe; 1) Umwandlung der Säuren aus der Milchsäure-Reihe in die der Acrylsäure-Reihe". Justus Liebigs Annalen der Chemie 136: 12. doi:10.1002/jlac.18651360102. https://zenodo.org/record/1427247.
 |

