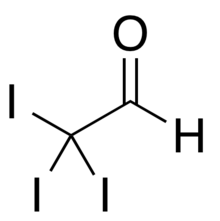
Chemistry:Iodal
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
triiodoethanal
| |
| Other names
triiodoacetaldehyde
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C2HI3O | |
| Molar mass | 421.742 g·mol−1 |
| Appearance | pale yellow liquid or solid |
| reacts to form a soluble hydrate | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Iodal, or triiodoacetaldehyde, is a halogenated derivative of acetaldehyde with the chemical formula I
3CCHO, it is analogous to chloral and bromal. It is described as a pale yellow liquid with a pungent odour by Leopold Gmelin. It is decomposed to iodoform by potash.[1] Iodal was discovered and named in 1837.[2]
Iodal is synthesised from ethanol and iodine with concentrated nitric acid as the catalyst.[1] Its hydrate was described as water-soluble, white silky crystals.[3] Like most iodine compounds, Iodal is unstable under sunlight and gives off iodine over time.
References
- ↑ 1.0 1.1 Iodal, Gmelin, L. (1855). Hand-book of Chemistry: Organic chemistry. UK: Cavendish Society. pages 186-187
- ↑ "Iodal" in Intelligence and Miscellaneous Articles, London and Edinburgh Philosophical Magazine and Journal of Science (1837)
- ↑ Iodal in Chemical Notices from Foreign Sources, The Chemical News: With which is Incorporated the Chemical Gazette: a Journal of Practical Chemistry in All Its Applications to Pharmacy, Arts, and Manufactures. (1862). UK: C. Mitchell and Company.
 |
