Chemistry:Irofulven
| |
| Names | |
|---|---|
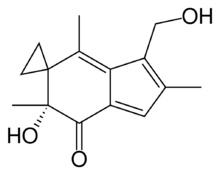
| Preferred IUPAC name
(6′R)-6′-Hydroxy-3′-(hydroxymethyl)-2′,4′,6′-trimethylspiro[cyclopropane-1,5′-inden]-7′(6′H)-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H18O3 | |
| Molar mass | 246.302 g/mol |
| Density | 1.285 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Irofulven or 6-hydroxymethylacylfulvene (also known as HMAF of MGI-114) is an experimental antitumor agent.[1][2] It belongs to the family of drugs called alkylating agents.
It inhibits the DNA replication of cells deficient in nucleotide excision repair in culture.[3][4]
Irofulven is an analogue of illudin S, a sesquiterpene toxin found in the Jack 'o' Lantern mushroom (Omphalotus illudens). The compound was originally synthesized by Dr. Trevor McMorris and found to have anticancer properties in mice by Dr. Michael J Kelner.[5]
Licensing and Clinical development
The drug was created and patented by the University of California, San Diego (UCSD), and subsequently licensed to the US biotech company MGI Pharma. Eisai acquired MGI in 2007, and the license was returned to UCSD, which then re-licensed the potential cancer drug to Lantern Pharma in 2015. Soon after, the drug was again sub-licensed to Oncology Venture.
The drug has undergone a number of clinical trials, mostly for late-stage tumors as well as ovarian and prostate cancers, usually preceded by treatment with carboplatin and paclitaxel. A multi-center phase 2 trial involving patients with Recurrent or Persistent Ovarian Epithelial or Primary Peritoneal Cancer was well tolerated but irofluven demonstrated modest activity as a single agent.[6] Previously, a European Phase I study in combination with cisplatin showed substantial evidence for anti-tumor activity. In that study, irofulven showed rapid elimination and high interpatient variability. Platinum and irofulven pharmacokinetics did not suggest drug-drug interactions.[7]
Despite modest successes demonstrating limited efficacy for late stage tumors that were statistically not significant enough to support broader clinical trials, Oncology Venture has decided to stratify patient populations with companion diagnostic tools (biomarkers) to predict outcomes, and thus select that sub-set of patients through DRP (Drug Response Predictors), for whom treatment with irofulven would be most effective. Oncology Venture Sweden AB has initiated two Phase 2 drug-screening studies at two Danish University Hospitals for late-stage prostate cancers, wherein 300 patients have been included to be screened, of which only 27 are to be selected for the Phase 2 trial.[8] This clinical trial is still ongoing with an expected completion date of mid 2024.[9]
Since it was first synthesized, research with irofulven has decreased dramatically over the past decade with only one clinical trial currently being run.[9] A study published in 2021 showed cells with nucleotide excision repair (NER) deficiencies were susceptible to iroflaven while cells without these NER deficiencies were not overly affected. The deficiency of tumors in NER was seen as a potential identifier for patient candidates.[4] Since this discovery, research has increased with researchers calling irofulven a "previously abandoned" anticancer drug.[10][4][11]
Synthesis
A synthesis of irofulven has been reported.[12]
References
- ↑ Escargueil, A. E.; Poindessous, V.; Soares, D. G.; Sarasin, A.; Cook, P. R.; Larsen, A. K. (April 2008). "Influence of Irofulven, a Transcription-Coupled Repair-Specific Antitumor Agent, on RNA Polymerase Activity, Stability and Dynamics in Living Mammalian Cells". Journal of Cell Science 121 (Pt 8): 1275–1283. doi:10.1242/jcs.023259. PMID 18388315.
- ↑ Kelner, M. J.; McMorris, T. C.; Estes, L.; Wang, W.; Samson, K. M.; Taetle, R. (1996). "Efficacy of HMAF (MGI-114) in the MV522 Metastatic Lung Carcinoma Xenograft Model Nonresponsive to Traditional Anticancer Agents". Investigational New Drugs 14 (2): 161–167. doi:10.1007/BF00210787. PMID 8913837.
- ↑ Wang, Y.; Wiltshire, T.; Senft, J.; Reed, E.; Wang, W. (February 2007). "Irofulven Induces Replication-Dependent CHK2 Activation Related to p53 Status". Biochemical Pharmacology 73 (4): 469–480. doi:10.1016/j.bcp.2006.10.023. PMID 17118344.
- ↑ 4.0 4.1 4.2 Börcsök, Judit; Sztupinszki, Zsofia; Bekele, Raie; Gao, Sizhi P.; Diossy, Miklos; Samant, Amruta S.; Dillon, Kasia M.; Tisza, Viktoria et al. (2021-04-01). "Identification of a Synthetic Lethal Relationship between Nucleotide Excision Repair Deficiency and Irofulven Sensitivity in Urothelial Cancer". Clinical Cancer Research: An Official Journal of the American Association for Cancer Research 27 (7): 2011–2022. doi:10.1158/1078-0432.CCR-20-3316. ISSN 1557-3265. PMID 33208343. PMC 8026514. https://pubmed.ncbi.nlm.nih.gov/33208343/.
- ↑ MacDonald, J. R.; Muscoplat, C. C.; Dexter, D. L.; Mangold, G. L.; Chen, S. F.; Kelner, M. J.; McMorris, T. C.; Von Hoff, D. D. (1997). "Preclinical Antitumor Activity of 6-hydroxymethylacylfulvene, a Semisynthetic Derivative of the Mushroom Toxin Illudin S". Cancer Research 57 (2): 279–283. PMID 9000568. http://cancerres.aacrjournals.org/content/57/2/279.full.pdf.
- ↑ Schilder RJ et al. (2010 Oct). "A phase II evaluation of Irofulven (IND#55804, NSC#683863) as second-line treatment of recurrent or persistent intermediately platinum-sensitive ovarian or primary peritoneal cancer: A Gynecologic Oncology Group trial". J Gynecol Cancer. 20(7):1137-41. PMC 3079178.
- ↑ Hilger W et al.(July 2006),A phase I and pharmacokinetic study of irofulven and cisplatin administered in a 30-min infusion every two weeks to patients with advanced solid tumors. Invest New Drugs. 24(4):311-9. doi:10.1007/s10637-005-5055-6.
- ↑ Both Danish sites now open in the Screening Study of prostate cancer patients for OV's Irofulven Pharmacy Choice - Pharmaceutical News. Retrieved 12 May 2017.
- ↑ 9.0 9.1 "ClinicalTrials.gov". https://clinicaltrials.gov/study/NCT03643107.
- ↑ Prosz, Aurel; Duan, Haohui; Tisza, Viktoria; Sahgal, Pranshu; Topka, Sabine; Klus, Gregory T.; Börcsök, Judit; Sztupinszki, Zsofia et al. (2023-11-23). "Nucleotide excision repair deficiency is a targetable therapeutic vulnerability in clear cell renal cell carcinoma" (in en). Scientific Reports 13 (1): 20567. doi:10.1038/s41598-023-47946-4. ISSN 2045-2322. PMC 10667362. https://www.nature.com/articles/s41598-023-47946-4.
- ↑ "Repairing DNA Damage in Cancer Cells | Memorial Sloan Kettering Cancer Center" (in en). 2021-01-11. https://www.mskcc.org/news/repairing-dna-damage-cancer-cells.
- ↑ McMorris, T. C.; Staake, M. D.; Kelner, M. J. (2004). "Synthesis and Biological Activity of Enantiomers of Antitumor Irofulven". The Journal of Organic Chemistry 69 (3): 619–23. doi:10.1021/jo035084j. PMID 14750783.
 |


