Chemistry:Isoglobotriosylceramide
| |
| Names | |
|---|---|
| IUPAC name
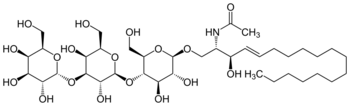
N-{(2S,3R,4E)-1-[α-D-Galactopyranosyl-(1→3)-β-D-galactopyranosyl-(1→4)-β-D-glucopyranosyloxy]-3-hydroxyoctadec-4-en-2-yl}acetamide
| |
| Systematic IUPAC name
N-[(2S,3R,4E)-1-{[(2R,3R,4R,5S,6R)-5-{[(2S,3R,4S,5S,6R)-3,5-dihydroxy-6-(hydroxymethyl)-4-{[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxan-2-yl]oxy}-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3-hydroxyoctadec-4-en-2-yl]acetamide | |
| Other names
isoglobotriasyl ceramide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
| C38H69NO18 | |
| Molar mass | 827.959 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Isoglobotriosylceramide, Gal(α1→3)Gal(β1→4)Glcβ(1→1)Cer, abbreviated as iGb3, is an iso-globo-series of glycosphingolipid, which mysteriously disappeared in most mammals studied (pig, mouse, and human), except trace amount reported in the thymus.[1]
iGb3 was discovered in canine[2][3] and rat intestines[4] among iso-globo-series of glycosphingolipids. First NMR spectrums for standard iGb3 were published by Dr. Tomoya Ogawa.[5][6]
The physiological function of iGb3 is not clear. It has been identified as a CD1d- presented self-antigen for an innate type of immune cells termed as Natural Killer T (NKT) cells.[7][8][9][10] Extensive biochemical studies by multiple methods including HPLC, mass spectrometry, and NMR did not lead to positive finding of iGb3 in major organs of mouse, pig, and human species,[11][12][13][14][15] except trace amount in thymus and immune cells, suggesting a selection pressure during evolution. Obviously, the immune selection pressure against iGb3 is mechanistically different from the well known anti-alpha-Gal antibodies, which caused the loss of alpha1,3-galactose epitope on glycoproteins in humans, apes, and old world monkeys.[16] The disappearance of iGb3 in pig and mouse species cannot be attributed to anti-alpha-Gal antibodies which are absent in these animals.
References
- ↑ "Lack of iGb3 and Isoglobo-Series Glycosphingolipids in Pig Organs Used for Xenotransplantation:Implications for Natural Killer T-Cell Biology". 28 April 2013. http://globalmedicaldiscovery.com/key-scientific-articles/lack-of-igb3-and-isoglobo-series-glycosphingolipids-in-pig-organs-used-for-xenotransplantationimplications-for-natural-killer-t-cell-biology/. Retrieved 3 June 2014.
- ↑ Sung SS, Sweeley CC. The structure of canine intestinal trihexosylceramide. Biochim Biophys Acta. 1979 Nov 21;575(2):295-98.
- ↑ Hansson GC, Karlsson KA, Larson G, McKibbin JM, Strömberg N, Thurin J. Isoglobotriaosylceramide and the Forssman glycolipid of dog small intestine occupy separate tissue compartments and differ in ceramide composition. Biochim Biophys Acta. 1983 Jan 7;750(1):214-6.
- ↑ Breimer ME, Hansson GC, Karlsson KA, Leffler H. Glycosphingolipids of rat tissues. Different composition of epithelial and nonepithelial cells of small intestine. J Biol Chem. 1982 Jan 10;257(1):557-68.
- ↑ Dabrowski J, Trauner K, Koike K, Ogawa T. Complete 1H-NMR spectral assignments for globotriaosyl-Z- and isoglobotriaosyl-E-ceramide. Chem Phys Lipids. 1988 Nov;49(1-2):31-7.
- ↑ Poppe L, Dabrowski J, von der Lieth CW, Koike K, Ogawa T. Three-dimensional structure of the oligosaccharide terminus of globotriaosylceramide and isoglobotriaosylceramide in solution. A rotating-frame NOE study using hydroxyl groups as long-range sensors in conformational analysis by 1H-NMR spectroscopy. Eur. J. Biochem. 1990 Apr 30;189(2):313-25.
- ↑ Zhou D, Mattner J, Cantu C 3rd, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, Teneberg S, Wang D, Proia RL, Levery SB, Savage PB, Teyton L, Bendelac A. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004 Dec 3;306(5702):1786-9.
- ↑ Zhou D. The immunological function of iGb3. Curr Protein Pept Sci. 2006 Aug;7(4):325-33.
- ↑ Schümann J, Mycko MP, Dellabona P, Casorati G, MacDonald HR. Cutting edge: influence of the TCR Vbeta domain on the selection of semi-invariant NKT cells by endogenous ligands. J Immunol. 2006 Feb 15;176(4):2064-8.
- ↑ Wei DG, Curran SA, Savage PB, Teyton L, Bendelac A. Mechanisms imposing the Vbeta bias of Valpha14 natural killer T cells and consequences for microbial glycolipid recognition. J Exp Med. 2006 May 15;203(5):1197-207.
- ↑ Speak AO, Salio M, Neville DC, Fontaine J, Priestman DA, Platt N, Heare T, Butters TD, Dwek RA, Trottein F, Exley MA, Cerundolo V, Platt FM. Implications for invariant natural killer T cell ligands due to the restricted presence of isoglobotrihexosylceramide in mammals. Proc Natl Acad Sci U S A. 2007 Apr 3;104(14):5971-6.
- ↑ Li Y, Thapa P, Hawke D, Kondo Y, Furukawa K, Furukawa K, Hsu FF, Adlercreutz D, Weadge J, Palcic MM, Wang PG, Levery SB, Zhou D. Immunologic glycosphingolipidomics and NKT cell development in mouse thymus. J Proteome Res. 2009 Jun;8(6):2740-51
- ↑ Puga Yung GL, Li Y, Borsig L, Millard AL, Karpova MB, Zhou D, Seebach JD. Complete absence of the !Gal xenoantigen and isoglobotrihexosylceramide in !1,3galactosyltransferase knockout pigs. Xenotransplantation. 2012 May-Jun;19(3):196-206
- ↑ Tahiri F, Li Y, Hawke D, Ganiko L, Almeida I, Levery S, Zhou D. Lack of iGb3 and Isoglobo-Series Glycosphingolipids in Pig Organs Used for Xenotransplantation: Implications for Natural Killer T-Cell Biology. J Carbohydr Chem. 2013 Jan 1;32(1):44-67.
- ↑ Porubsky S, Speak AO, Salio M, Jennemann R, Bonrouhi M, Zafarulla R, Singh Y, Dyson J, Luckow B, Lehuen A, Malle E, Müthing J, Platt FM, Cerundolo V, Gröne HJ. Globosides but not isoglobosides can impact the development of invariant NKT cells and their interaction with dendritic cells. J Immunol. 2012 Sep 15;189(6):3007-17.
- ↑ Macher BA, Galili U. The Galalpha1,3Galbeta1,4GlcNAc-R (alpha-Gal) epitope: a carbohydrate of unique evolution and clinical relevance. Biochim Biophys Acta. 2008 Feb;1780(2):75-88.
 |

