Chemistry:JNJ-26990990
From HandWiki
Short description: Experimental anticonvulsant drug
 | |
| Clinical data | |
|---|---|
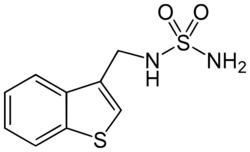
| Other names | N-((Benzo[b]thien-3-yl)methyl)sulfamide |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C9H10N2O2S2 |
| Molar mass | 242.31 g·mol−1 |
| | |
JNJ-26990990 is a broad-spectrum anticonvulsant drug currently under development by Janssen Pharmaceutica as a second-generation follow-up to the marketed drug topiramate.[1][2] It was designed to have the same anticonvulsant effects as topiramate, but without the side effects associated with topiramate's carbonic anhydrase inhibition.[1] It also has potential use in the treatment of inflammatory pain, neuropathic pain, and depression.[3]
JNJ-26990990 entered phase II clinical trials in October 2007.[4]
Metabolites and radioactive isotope-labeled derivatives of JNJ-26990990 have also been prepared to support its development.[5]
See also
References
- ↑ 1.0 1.1 "Novel, broad-spectrum anticonvulsants containing a sulfamide group: advancement of N-((benzo[b]thien-3-yl)methyl)sulfamide (JNJ-26990990) into human clinical studies". Journal of Medicinal Chemistry 52 (23): 7528–7536. December 2009. doi:10.1021/jm801432r. PMID 19388676.
- ↑ Parker MH, Reitz AB, Maryanoff BE, "Preparation of benzo-fused heteroaryl sulfamide derivatives for the treatment of epilepsy and related disorders", WO patent 2006023861, issued 2006-03-02, assigned to Janssen Pharmaceutica
- ↑ "JNJ 26990990 Johnson & Johnson developing anticonvulsant Johnson & Johnson preclinical data". R & D Focus Drug News. September 22, 2008. http://business.highbeam.com/436989/article-1G1-185376163/jnj-26990990-johnson-johnson-developing-anticonvulsant.
- ↑ "Chapter 2. Standalone Drugs". Analogue-Based Drug Discovery II. 2010. doi:10.1002/9783527630035.ch2.
- ↑ "Expeditious syntheses of stable and radioactive isotope-labeled anticonvulsant agent, JNJ-26990990, and its metabolites". Journal of Labelled Compounds & Radiopharmaceuticals 56 (1): 22–26. January 2013. doi:10.1002/jlcr.3013. PMID 24285137.
 |

