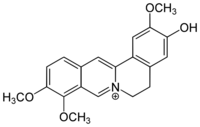
Chemistry:Jatrorrhizine
| |
| Names | |
|---|---|
| IUPAC name
3-Hydroxy-2,9,10-trimethoxy-7,8,13,13a-tetradehydroberbin-7-ium
| |
| Systematic IUPAC name
3-Hydroxy-2,9,10-trimethoxy-5,6-dihydro-7λ5-isoquinolino[3,2-a]isoquinolin-7-ylium | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C20H20NO4+1 | |
| Molar mass | 338.382 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Jatrorrhizine is a protoberberine alkaloid found in some plant species, such as Enantia chlorantha (Annonaceae).[1] Synonyms that may be encountered include jateorrhizine, neprotin, jatrochizine, jatrorhizine, and yatrorizine.
Bioactive effects
Jatrorrhizine has been reported to have antiinflammatory effect,[2] and to improve blood flow and mitotic activity in thioacetamide-traumatized rat livers.[3] It was found to have antimicrobial[4] and antifungal[5] activity. It binds and noncompetitively inhibits monoamine oxidase (IC50 = 4 μM for MAO-A and 62 μM for MAO-B)[6] It interferes with multidrug resistance by cancer cells in vitro when exposed to a chemotherapeutic agent.[7] Large doses (50–100 mg/kg) reduced blood sugar levels in mice by increasing aerobic glycolysis.
Derivatives of jatrorrhizine (notably 3-alkoxy derivatives, and specifically 3-octyloxy 8-alkyljatrorrhizine derivatives such as 3-octyloxy 8-butyljatrorrhizine) have been synthesized and found to have much stronger antimicrobial effects.[8][9][10]
References
- ↑ "jatrorrhizine - Compound Summary (CID 72323)". PubChem. https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=72323&loc=ec_rcs.
- ↑ Arens, H; Fischer, H; Leyck, S; Römer, A; Ulbrich, B (1985). "Antiinflammatory Compounds from Plagiorhegma dubium Cell Culture1". Planta Medica 51 (1): 52–6. doi:10.1055/s-2007-969392. PMID 17340402.
- ↑ Virtanen, P; Lassila, V; Njimi, T; Mengata, DE (1988). "Natural protoberberine alkaloids from Enantia chlorantha, palmatine, columbamine and jatrorrhizine for thioacetamide-traumatized rat liver". Acta Anatomica 131 (2): 166–70. doi:10.1159/000146507. PMID 3369286.
- ↑ Moody, JO; Bloomfield, SF; Hylands, PJ (1995). "In-vitro evaluation of the antimicrobial activities of Enantia chlorantha Oliv. Extractives". African Journal of Medicine and Medical Sciences 24 (3): 269–73. PMID 8798963.
- ↑ Volleková, A; Kost'álová, D; Kettmann, V; Tóth, J (2003). "Antifungal activity of Mahonia aquifolium extract and its major protoberberine alkaloids". Phytotherapy Research 17 (7): 834–7. doi:10.1002/ptr.1256. PMID 12916091.
- ↑ Kong, LD; Cheng, CH; Tan, RX (2001). "Monoamine oxidase inhibitors from rhizoma of Coptis chinensis". Planta Medica 67 (1): 74–6. doi:10.1055/s-2001-10874. PMID 11270727.
- ↑ Zhang, H; Yang, L; Liu, S; Ren, L (2001). "Study on active constituents of traditional Chinese medicine reversing multidrug resistance of tumor cells in vitro". Zhong Yao Cai 24 (9): 655–7. PMID 11799777.
- ↑ Wang, LJ; Ye, XL; Li, XG; Sun, QL; Yu, G; Cao, XG; Liang, YT; Zhang, HS et al. (2008). "Synthesis and antimicrobial activity of 3-alkoxyjatrorrhizine derivatives". Planta Medica 74 (3): 290–2. doi:10.1055/s-2008-1034312. PMID 18300191.
- ↑ Wang, LJ; Ye, XL; Chen, Z; Li, XG; Sun, QL; Zhang, BS; Cao, XG; Yu, G et al. (2009). "Synthesis and antimicrobial activity of 3-octyloxy-8-alkyljatrorrhizine derivatives". Journal of Asian Natural Products Research 11 (4): 365–70. doi:10.1080/10286020902727447. PMID 19431018.
- ↑ Bhadra, K; Kumar, GS (2010). "Therapeutic potential of nucleic acid-binding isoquinoline alkaloids: Binding aspects and implications for drug design". Medicinal Research Reviews 31 (6): 821–862. doi:10.1002/med.20202. PMID 20077560.
 |

