Biology:Monoamine oxidase
| Monoamine oxidase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 1.4.3.4 | ||||||||
| CAS number | 9001-66-5 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| Monoamine oxidase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | MAO | ||||||||
| Pfam | PF01593 | ||||||||
| InterPro | IPR001613 | ||||||||
| OPM superfamily | 119 | ||||||||
| OPM protein | 2z5x | ||||||||
| Membranome | 418 | ||||||||
| |||||||||
| monoamine oxidase A | |
|---|---|
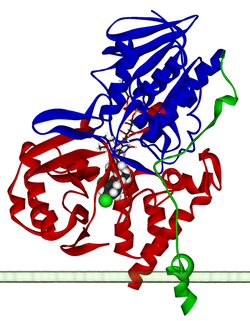 Ribbon diagram of a monomer of human MAO-A, with FAD and clorgiline bound, oriented as if attached to the outer membrane of a mitochondrion. From PDB: 2BXS. | |
| Identifiers | |
| Symbol | MAOA |
| NCBI gene | 4128 |
| HGNC | 6833 |
| OMIM | 309850 |
| RefSeq | NM_000240 |
| UniProt | P21397 |
| Other data | |
| Locus | Chr. X p11.4-p11.3 |
| monoamine oxidase B | |
|---|---|
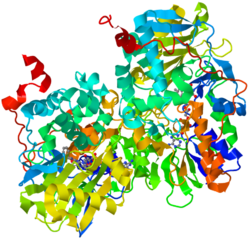 | |
| Identifiers | |
| Symbol | MAOB |
| NCBI gene | 4129 |
| HGNC | 6834 |
| OMIM | 309860 |
| RefSeq | NM_000898 |
| UniProt | P27338 |
| Other data | |
| Locus | Chr. X p11.4-p11.3 |
Monoamine oxidases (MAO) (EC 1.4.3.4) are a family of enzymes that catalyze the oxidation of monoamines, employing oxygen to clip off their amine group.[1][2] They are found bound to the outer membrane of mitochondria in most cell types of the body. The first such enzyme was discovered in 1928 by Mary Bernheim in the liver and was named tyramine oxidase.[3][4] The MAOs belong to the protein family of flavin-containing amine oxidoreductases.
MAOs are important in the breakdown of monoamines ingested in food, and also serve to inactivate monoamine neurotransmitters. Because of the latter, they are involved in a number of psychiatric and neurological diseases, some of which can be treated with monoamine oxidase inhibitors (MAOIs) which block the action of MAOs.[5]
Subtypes and tissue distribution
In humans there are two types of MAO: MAO-A and MAO-B.[6]
- Both are found in neurons and astroglia.
- Outside the central nervous system:
- MAO-A is also found in the liver, pulmonary vascular endothelium, gastrointestinal tract, and placenta.
- MAO-B is mostly found in blood platelets.
MAO-A appears at roughly 80% of adulthood levels at birth, increasing very slightly after the first 4 years of life, while MAO-B is almost non-detectable in the infant brain. Regional distribution of the monoamine oxidases is characterized by extremely high levels of both MAOs in the hypothalamus and hippocampal uncus, as well as a large amount of MAO-B with very little MAO-A in the striatum and globus pallidus. The cortex has relatively high levels of only MAO-A, with the exception of areas of the cingulate cortex, which contains a balance of both. Autopsied brains demonstrated the predicted increased concentration of MAO-A in regions dense in serotonergic neurotransmission, however MAO-B only correlated with norepinephrine.[7]
Other studies, in which the activities of MAO (not protein amounts) were examined in rat brain, revealed the highest MAO-B activity in the median eminence of hypothalamus. Dorsal raphe nucleus and medial preoptic area have relatively high MAO-B activity, but much lower than MAO-B activity in the median eminence.[8][9] Among cerebral endocrine glands, pineal gland has high MAO-B activity (its median value is lower than that for median eminence and higher than that for medial preoptic area).[9] Pituitary has the lowest level of MAO-B activity when compared with brain areas studied.[8]
Function
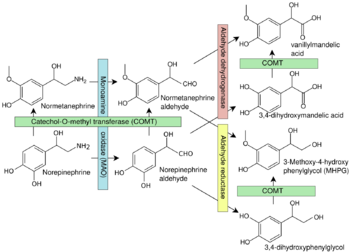
Monoamine oxidases catalyze the oxidative deamination of monoamines. In the first part of reaction, cofactor FAD oxidase substrate yielding corresponding imine which converts the cofactor into its reduced form FADH2. Imine is then non-enzymatically hydrolyzed to corresponding ketone (or aldehyde) and ammonia. Oxygen is used to restore reduced FADH2 cofactor back to the active FAD form. Monoamine oxidases contain the covalently bound cofactor FAD and are, thus, classified as flavoproteins. Monoamine oxidase A and B share roughly 70% of their structure and both have substrate binding sites that are predominantly hydrophobic. Two tyrosine residues (398, 435 within MAO-B, 407 and 444 within MAO-A) in the binding pocket that are commonly involved in inhibitor activity have been hypothesized to be relevant to orienting substrates, and mutations of these residues are relevant to mental health. Four main models have been proposed for the mechanism of electron transfer (single electron transfer, hydrogen atom transfer, nucleophilic model, and hydride transfer[11]) although there is insufficient evidence to support any of them.[12]
Substrate specificities
They are well known enzymes in pharmacology, since they are the target for the action of a number of monoamine oxidase inhibitor drugs. MAO-A is particularly important in the catabolism of monoamines ingested in food. Both MAOs are also vital to the inactivation of monoamine neurotransmitters, for which they display different specificities.
- Serotonin, melatonin, norepinephrine, and epinephrine are mainly broken down by MAO-A.
- Phenethylamine and benzylamine are mainly broken down by MAO-B.
- Both forms metabolize dopamine, tyramine, and tryptamine;[13] however, some evidence suggests MAO-B may not be responsible for a significant amount of dopamine degradation.[14]
Specific reactions catalyzed by MAO include:
- Adrenaline or noradrenaline to 3,4-Dihydroxymandelic acid
- Metanephrine or normetanephrine to vanillylmandelic acid (VMA)
- Dopamine to dihydroxyphenylacetic acid
- 3-Methoxytyramine to homovanillic acid
Clinical significance
Because of the vital role that MAOs play in the inactivation of neurotransmitters, MAO dysfunction (too much or too little MAO activity) is thought to be responsible for a number of psychiatric and neurological disorders. For example, unusually high or low levels of MAOs in the body have been associated with schizophrenia,[15][16] depression,[17] attention deficit disorder,[18] substance abuse,[19] migraines,[20][21] and irregular sexual maturation.[citation needed] Monoamine oxidase inhibitors are one of the major classes of drug prescribed for the treatment of depression, although they are often last-line treatment due to risk of the drug's interaction with diet or other drugs. Excessive levels of catecholamines (epinephrine, norepinephrine, and dopamine) may lead to a hypertensive crisis, and excessive levels of serotonin may lead to serotonin syndrome.
In fact, MAO-A inhibitors act as antidepressant and anti-anxiety agents, whereas MAO-B inhibitors are used alone or in combination to treat Alzheimer's disease and Parkinson's disease.[22] Some research suggests that certain phenotypes of depression, such as those with anxiety, and "atypical" symptoms involving psychomotor retardation, weight gain and interpersonal sensitivity respond better to MAO inhibitors than other classes of anti-depressant. However the findings related to this have not been consistent.[23] MAOIs may be effective in treatment resistant depression, especially when it does not respond to tricyclic antidepressants.[24]
Parasite interactions
Sleeping sickness - caused by trypanosomes - gets its name from the sleep disruption it causes in mammals. That sleep disruption is caused, at least in part, by trypanosomes' tendency to disrupt MAO activity in the orexin system.[25]
Animal models
There are significant differences in MAO activity in different species. Dopamine is primarily deaminated by MAO-A in rats, but by MAO-B in vervet monkeys and humans.[26]
Mice unable to produce either MAO-A or MAO-B display autistic-like traits.[27] These knockout mice display an increased response to stress.[28]
Arthropods
Insects
Insect brains express MAOs,[29][30][31] and some insecticides[32][31] work by inhibiting them. An MAOI effect is especially important for chlordimeform[32][31][33] (although one result shows little or no effect in Periplaneta americana);[34] and dieldrin may[29] or may not[30] be an MAOI in Locusta migratoria.
Acari
MAO activity has been detected in Rhipicephalus microplus and chlordimeform is an MAOI in R. m..[35]
Genetics
The genes encoding MAO-A and MAO-B are located side-by-side on the short arm of the X chromosome, and have about 70% sequence similarity. Rare mutations in the gene are associated with Brunner syndrome.
A study based on the Dunedin cohort concluded that maltreated children with a low-activity polymorphism in the promoter region of the MAO-A gene were more likely to develop antisocial conduct disorders than maltreated children with the high-activity variant.[36] Out of the 442 total males in the study (maltreated or not), 37% had the low activity variant. Of the 13 maltreated males with low MAO-A activity, 11 had been assessed as exhibiting adolescent conduct disorder and 4 were convicted for violent offenses. The suggested mechanism for this effect is the decreased ability of those with low MAO-A activity to quickly degrade norepinephrine, the synaptic neurotransmitter involved in sympathetic arousal and rage. This is argued to provide direct support for the idea that genetic susceptibility to disease is not determined at birth, but varies with exposure to environmental influences. However, most individuals with conduct disorder or convictions did not have low activity of MAO-A; maltreatment was found to have caused stronger predisposition for antisocial behavior than differences in MAO-A activity.
The claim that an interaction between low MAO-A activity and maltreatment would cause anti-social behavior has been criticized since the predisposition towards anti-social behavior could equally well have been caused by other genes inherited from abusive parents.[37]
A possible link between predisposition to novelty seeking and a genotype of the MAO-A gene has been found.[38]
A particular variant (or genotype), dubbed "warrior gene" in the popular press, was over-represented in Māori. This supported earlier studies finding different proportions of variants in different ethnic groups. This is the case for many genetic variants, with 33% White/Non-Hispanic, 61% Asian/Pacific Islanders having the low-activity MAO-A promoter variant.[39]
Aging
Unlike many other enzymes, MAO-B activity is increased during aging in the brain of humans and other mammals.[40] Increased MAO-B activity was also found in the pineal gland of aging rats.[9] This may contribute to lowered levels of monoamines in aged brain and pineal gland.[9][41]
See also
- Cheese effect
- I2 receptor
- Monoamine oxidase inhibitor
References
- ↑ "Monoamine oxidases: certainties and uncertainties". Current Medicinal Chemistry 11 (15): 1965–82. August 2004. doi:10.2174/0929867043364810. PMID 15279561.
- ↑ "Structure and mechanism of monoamine oxidase". Current Medicinal Chemistry 11 (15): 1983–93. August 2004. doi:10.2174/0929867043364784. PMID 15279562.
- ↑ "Tyramine oxidase: A new enzyme system in liver". The Biochemical Journal 22 (4): 968–79. 1928. doi:10.1042/bj0220968. PMID 16744124.
- ↑ "Mary Bernheim and the discovery of monoamine oxidase". Brain Research Bulletin 50 (5–6): 373. 1999. doi:10.1016/S0361-9230(99)00110-0. PMID 10643441.
- ↑ "Monoamine Oxidases (MAOs) as Privileged Molecular Targets in Neuroscience: Research Literature Analysis". Frontiers in Molecular Neuroscience 12: 143. 2019. doi:10.3389/fnmol.2019.00143. PMID 31191248.
- ↑ "Regulation of MAO-A and MAO-B gene expression". Current Medicinal Chemistry 11 (15): 1995–2005. August 2004. doi:10.2174/0929867043364757. PMID 15279563.
- ↑ "Distribution of monoamine oxidase proteins in human brain: implications for brain imaging studies". Journal of Cerebral Blood Flow and Metabolism 33 (6): 863–71. June 2013. doi:10.1038/jcbfm.2013.19. PMID 23403377.
- ↑ 8.0 8.1 "Monoamine oxidase activity in several structures of rat brain". Neurochemical Journal 1 (3): 204–207. 2007-09-01. doi:10.1134/S1819712407030051.
- ↑ 9.0 9.1 9.2 9.3 "Monoamine oxidase activity in the rat pineal gland: Comparison with brain areas and alteration during aging". Advances in Gerontology 6 (2): 111–116. 2016-04-01. doi:10.1134/S2079057016020120.
- ↑ Figure 11-4 in: Rang & Dale's pharmacology. Edinburgh: Churchill Livingstone. 2007. ISBN 978-0-443-06911-6.
- ↑ "How are Biogenic Amines Metabolized by Monoamine Oxidases?". European Journal of Organic Chemistry 2012 (36): 7057–7065. 2012-10-25. doi:10.1002/ejoc.201201122.
- ↑ "Structures and Mechanism of the Monoamine Oxidase Family". Biomolecular Concepts 2 (5): 365–377. October 2011. doi:10.1515/BMC.2011.030. PMID 22022344.
- ↑ "Interactions of nitrogen-containing xenobiotics with monoamine oxidase (MAO) isozymes A and B: SAR studies on MAO substrates and inhibitors". Chemical Research in Toxicology 14 (9): 1139–62. September 2001. doi:10.1021/tx010073b. PMID 11559028.
- ↑ "Redefining differential roles of MAO-A in dopamine degradation and MAO-B in tonic GABA synthesis". Exp Mol Med 53 (7): 1148–1158. July 2021. doi:10.1038/s12276-021-00646-3. PMID 34244591.
- ↑ "Decreased blood platelet MAO activity in unmedicated chronic schizophrenic patients". The American Journal of Psychiatry 133 (3): 323–6. March 1976. doi:10.1176/ajp.133.3.323. PMID 943955.
- ↑ "Reduced platelet monoamine oxidase activity in a subgroup of schizophrenic patients". The American Journal of Psychiatry 133 (4): 438–40. April 1976. doi:10.1176/ajp.133.4.438. PMID 1267046.
- ↑ "Elevated monoamine oxidase a levels in the brain: an explanation for the monoamine imbalance of major depression". Archives of General Psychiatry 63 (11): 1209–16. November 2006. doi:10.1001/archpsyc.63.11.1209. PMID 17088501.
- ↑ "Association analysis of the monoamine oxidase A and B genes with attention deficit hyperactivity disorder (ADHD) in an Irish sample: preferential transmission of the MAO-A 941G allele to affected children". American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics 134B (1): 110–4. April 2005. doi:10.1002/ajmg.b.30158. PMID 15717295.
- ↑ "Platelet monoamine oxidase, personality and alcoholism: the rise, fall and resurrection". Neurotoxicology 25 (1–2): 79–89. January 2004. doi:10.1016/S0161-813X(03)00115-3. PMID 14697883.
- ↑ "Monoamine oxidase activities in patients with migraine or with cluster headache during the acute phases and after treatment with L-5-hydroxytryptophan". Rivista di Patologia Nervosa e Mentale 100 (5): 269–74. 1 October 2016. PMID 318025.
- ↑ "Monoamine oxidases A and B gene polymorphisms in migraine patients". Journal of the Neurological Sciences 228 (2): 149–53. February 2005. doi:10.1016/j.jns.2004.11.045. PMID 15694196.
- ↑ "Clinical applications of MAO-inhibitors". Current Medicinal Chemistry 11 (15): 2033–43. August 2004. doi:10.2174/0929867043364775. PMID 15279566.
- ↑ "The clinical characterization of the adult patient with depression aimed at personalization of management". World Psychiatry 19 (3): 269–293. October 2020. doi:10.1002/wps.20771. PMID 32931110.
- ↑ "The role of monoamine oxidase inhibitors in current psychiatric practice". Journal of Psychiatric Practice 10 (4): 239–48. July 2004. doi:10.1097/00131746-200407000-00005. PMID 15552546.
- ↑ "African trypanosome infections of the nervous system: parasite entry and effects on sleep and synaptic functions". Progress in Neurobiology 91 (2): 152–71. June 2010. doi:10.1016/j.pneurobio.2009.12.001. PMID 19995590.
- ↑ "Species differences in the deamination of dopamine and other substrates for monoamine oxidase in brain". Psychopharmacology 72 (1): 27–33. 1980. doi:10.1007/bf00433804. PMID 6781004.
- ↑ "Monoamine oxidase A and A/B knockout mice display autistic-like features". The International Journal of Neuropsychopharmacology 16 (4): 869–88. May 2013. doi:10.1017/S1461145712000715. PMID 22850464.
- ↑ "Cloning, after cloning, knock-out mice, and physiological functions of MAO A and B". Neurotoxicology 25 (1–2): 21–30. January 2004. doi:10.1016/s0161-813x(03)00112-8. PMID 14697877.
- ↑ 29.0 29.1 "Sur l'activité monoaminoxydasique du cerveau de Locusta migratoria dans les conditions normales et après intoxication par deux insecticides: le chlordiméform et la diéldrine". Comptes Rendus de l'Académie des Sciences, Série D 284 (12): 1079–82. March 1977. PMID 406057.
- ↑ 30.0 30.1 "Biogenic Amines in the Insect Nervous System". Advances in Insect Physiology. 15. 1980. pp. 317–473. doi:10.1016/s0065-2806(08)60143-5. ISBN 978-0-12-024215-3.
- ↑ 31.0 31.1 31.2 "Chlordimeform: Plant protection by a sublethal, noncholinergic action on the central nervous system". Pesticide Biochemistry and Physiology 11 (1–3): 117–128. 1979. doi:10.1016/0048-3575(79)90052-x. ISSN 0048-3575.
- ↑ 32.0 32.1 "Inhibition of monoamine oxidase by the pesticide chlordimeform and related compounds". Nature 242 (5397): 417–8. April 1973. doi:10.1038/242417a0. PMID 4701207. Bibcode: 1973Natur.242..417A.
- ↑ "Studies on the action of chlordimeform in cockroaches". Pesticide Biochemistry and Physiology 4 (3): 325–336. 1974. doi:10.1016/0048-3575(74)90115-1. ISSN 0048-3575.
- ↑ "Effects of chlordimeform and lindane on monoamine levels in the central nervous system of the american cockroach, Periplaneta americana L.". Pesticide Biochemistry and Physiology 24 (2): 213–219. 1985. doi:10.1016/0048-3575(85)90131-2. ISSN 0048-3575.
- ↑ P. Atkinson; K. Binnington; W. J. Roulston (1974). "High monoamine oxidase activity in the tick Boophilus Microplus and inhibition by chlordimeform and related pesticides". Australian Journal of Entomology 13 (3): 207–210. doi:10.1111/j.1440-6055.1974.tb02174.x.
- ↑ "Role of genotype in the cycle of violence in maltreated children". Science 297 (5582): 851–4. August 2002. doi:10.1126/science.1072290. PMID 12161658. Bibcode: 2002Sci...297..851C.
- ↑ Making sense of heritability. Cambridge, UK: Cambridge University Press. 2005. ISBN 978-0-521-82818-5.
- ↑ "Monoamine oxidase A gene promoter polymorphism affects novelty seeking and reward dependence in healthy study participants". Psychiatric Genetics 16 (2): 55–8. April 2006. doi:10.1097/01.ypg.0000199447.62044.ef. PMID 16538181.
- Heidi Dawley (June 18, 2006). "The disorder of these times, neophilia". Media Life. http://www.medialifemagazine.com/cgi-bin/artman/exec/view.cgi?archive=226&num=5439.
- ↑ "A functional polymorphism in the monoamine oxidase A gene promoter". Human Genetics 103 (3): 273–9. September 1998. doi:10.1007/s004390050816. PMID 9799080. https://zenodo.org/record/1232725.
- ↑ "Monoamine oxidase expression during development and aging". Neurotoxicology 25 (1–2): 155–65. January 2004. doi:10.1016/S0161-813X(03)00095-0. PMID 14697890.
- ↑ "[Pineal gland and brain structures monoamine oxidase activity in rats of different age]" (in Russian). Advances in Gerontology = Uspekhi Gerontologii 21 (3): 402–5. 2008. PMID 19432173.
 |

