Chemistry:Lactamide
From HandWiki
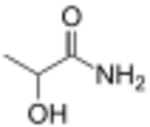
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Hydroxypropanamide | |
| Other names
2-Hydroxypropionamide; Lactic acid amide; Lactic amide; α-Hydroxypropionamide
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C3H7NO2 | |
| Molar mass | 89.094 g·mol−1 |
| Melting point | 73 to 76 °C (163 to 169 °F; 346 to 349 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Lactamide is an amide derived from lactic acid. It is a white crystalline solid with a melting point of 73-76 °C.
Lactamide can be prepared by the catalytic hydration of lactonitrile.[2]
References
- ↑ Chemical Book Lactamide
- ↑ Fumio Tanaka, Tsumoru Morimoto, Takako Uchiyama, Takafumi Abe, "Process for preparing lactamide", US patent 5756842, issued 1998-05-26
 |
