Chemistry:Lactimidomycin
From HandWiki
Short description: Chemical compound
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
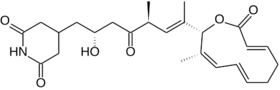
| Formula | C26H35NO6 |
| Molar mass | 457.558 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Lactimidomycin is a glutarimide antibiotic derived from the bacteria Streptomyces amphibiosporus.[1][2] It has antifungal, antiviral and anti-cancer properties, acting as a direct inhibitor of protein translation in ribosomes.[3][4][5] Antiviral activity is seen against a variety of RNA viruses including flaviviruses such as dengue fever, Kunjin virus and Modoc virus, as well as vesicular stomatitis virus and poliovirus.[6] As lactimidomycin is a natural product containing an unusual unsaturated 12-membered lactone ring, it has been the subject of numerous total synthesis approaches.[7][8][9][10]
References
- ↑ "Lactimidomycin, a new glutarimide group antibiotic. Production, isolation, structure and biological activity". The Journal of Antibiotics 45 (9): 1433–41. September 1992. doi:10.7164/antibiotics.45.1433. PMID 1429229.
- ↑ "New lactimidomycin congeners shed insight into lactimidomycin biosynthesis in Streptomyces amphibiosporus". Organic Letters 9 (25): 5183–6. December 2007. doi:10.1021/ol702249g. PMID 17997563.
- ↑ "Inhibition of eukaryotic translation elongation by cycloheximide and lactimidomycin". Nature Chemical Biology 6 (3): 209–217. March 2010. doi:10.1038/nchembio.304. PMID 20118940.
- ↑ "Total syntheses and biological reassessment of lactimidomycin, isomigrastatin and congener glutarimide antibiotics". Chemistry: A European Journal 19 (23): 7370–83. June 2013. doi:10.1002/chem.201300393. PMID 23595541.
- ↑ "Structural basis for the inhibition of the eukaryotic ribosome". Nature 513 (7519): 517–22. September 2014. doi:10.1038/nature13737. PMID 25209664. Bibcode: 2014Natur.513..517G.
- ↑ "Lactimidomycin is a broad-spectrum inhibitor of dengue and other RNA viruses". Antiviral Research 128: 57–62. April 2016. doi:10.1016/j.antiviral.2016.02.005. PMID 26872864.
- ↑ "Concise total synthesis of the potent translation and cell migration inhibitor lactimidomycin". Journal of the American Chemical Society 132 (40): 14064–6. October 2010. doi:10.1021/ja107141p. PMID 20831202.
- ↑ "Synthesis of eukaryotic translation elongation inhibitor lactimidomycin via Zn(II)-mediated Horner-Wadsworth-Emmons macrocyclization". Organic Letters 15 (12): 2998–3001. June 2013. doi:10.1021/ol401186f. PMID 23731327.
- ↑ "Formal total synthesis of lactimidomycin". Organic Letters 15 (12): 3002–5. June 2013. doi:10.1021/ol401214f. PMID 23731346.
- ↑ "Synthesis and Biological Evaluation of Lactimidomycin and Its Analogues". Chemistry: A European Journal 21 (52): 19159–67. December 2015. doi:10.1002/chem.201503527. PMID 26577990.
 |

