Chemistry:Lanthanocene
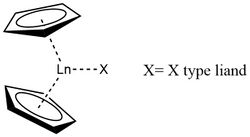
A lanthanocene is a type of metallocene compound that contains an element from the lanthanide series. The most common lanthanocene complexes contain two cyclopentadienyl anions and an X type ligand, usually hydride or alkyl ligand.
History
In 1954, Wilkinson and Birmingham described the tris(cyclopentadienyl)lanthanide complex, Ln(C5H5)3 (Ln = La, Ce, Pr, Nd, Sm).[1][2] However, due to the highly moisture and oxygen sensitive character of organolanthanide compounds, as well as the incapability of the separation of simple alkyl and aryl derivatives of the type LnR3, this area of organometallic chemistry experienced a period of relative stagnation for two decades before lanthanocene complexes were prepared for some of the later lanthanides (Ln = Gd, Er, Yb, Lu).[3][4][5][6]
Synthesis
The synthesis part will focus on lanthanide(III) metallocene complexes that contain Ln-C bonds, which are widely prepared from the corresponding Ln-Cl precursors as shown.[3]
- (C
5H
5)
2LnCl(THF) + LiR → (C
5H
5)
2LnR(THF) + LiCl- Ln = Y, Nd, Sm, Dy, Er, Tm, Yb, Lu
- R = Me, Et, iPr, nBu, tBu, CH2tBu, CH
2SiMe
3, CH
2Ph, Ph, C
6H
4Me−
p
The synthetic route leading to lanthanocene chlorides are summarized:
- LnCl
3 + 2 MC
5H
5 → (C
5H
5)
2LnCl + 2 MCl- M = Na, Ti
- Ln(C
5H
5)
3 + NH
4Cl → (C
5H
5)
2LnCl + C
5H
6 + NH
3 - Ln(C
5H
5)
3 + HCl → (C
5H
5)
2LnCl + C
5H
6
Reactions
With the large 4f orbitals, lanthanide elements display properties significantly different from the common d-block transition metals. The large ionic radii limits the extent to which 4f orbitals can overlap with ligands, but at the same time allows the organolanthanide complexes to attain higher coordination numbers.[5] As such, cyclopentadienyl anions (abbreviated Cp) are typically used to occupy the unsaturated site as well as stabilize the metal complex. Some of the alkyl and hydride lanthanocene complexes exhibit unique activities towards C-H bond activation,[6] alkene functionalization,[7] and carbonyl activation.[8]
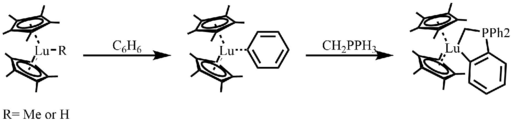
In 1983, Watson reported one of the first lanthanocene catalyzed C-H bond activation reactions.[6] The active catalysts are lutetium-methyl or lutetium-hydride complexes, which react at room temperature in hydrocarbon solvents with benzene, pyridine, and the ylide CH2PPh3 to give stable, isolatable products.
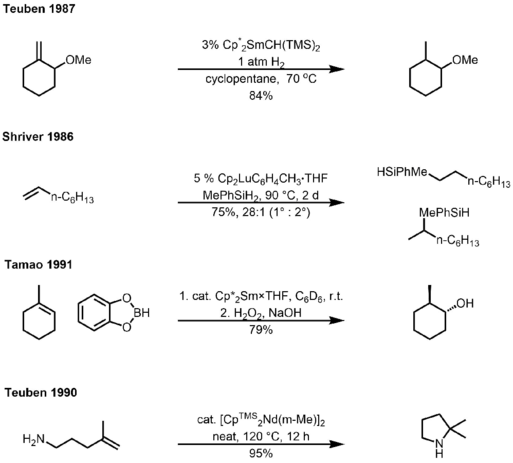
Studies have shown that organolanthanides are extraordinary catalysts for hydrofunctionalization reactions including hydrogenation, hydrosilylation, hydroboration, hydroamination, etc. Examples for each type have shown below.[9][10][11][12]
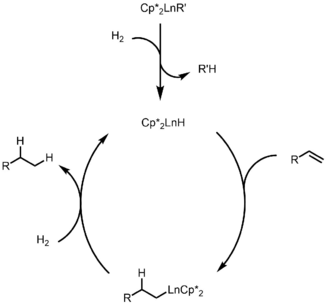
Mechanism for hydrogenation is shown below, where the active catalyst is generated by sigma-bond metathesis, followed by olefin insertion and another sigma-bond metathesis to regenerate the catalyst. This is also the mechanism for other hydrofunctionalization.
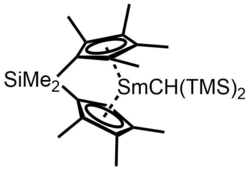
Yet, as the large cyclopentadienyl ligand hinders the metal center, reactions with substituted alkenes are inhibited. Two general methods are used to overcome this difficulty. One is to increase the size of metal by incorporating lanthanides with larger ionic radii. Due to the lanthanide contraction,[13] this means replacing the late lanthanides with the early lanthanides. Another method is to decrease the size of the ligand by manipulating the geometry of ligands and substitutions on ligands.[7] For example, a hinged or ansa-bridged cyclopentadienyl ligand could be used to pull ligands closer to each other, and hence creating more open access to the metal center as shown on the right.[14]
See also
References
- ↑ Wilkinson, G.; Birmingham, J. M. (December 1954). "CYCLOPENTADIENYL COMPOUNDS OF Sc, Y, La, Ce AND SOME LANTHANIDE ELEMENTS". Journal of the American Chemical Society 76 (23): 6210. doi:10.1021/ja01652a114. ISSN 0002-7863.
- ↑ Birmingham, J. M.; Wilkinson, G. (January 1956). "The Cyclopentadienides of Scandium, Yttrium and Some Rare Earth Elements". Journal of the American Chemical Society 78 (1): 42–44. doi:10.1021/ja01582a009. ISSN 0002-7863.
- ↑ 3.0 3.1 Edelmann, Frank T. (2008), "Lanthanocenes", Metallocenes, John Wiley & Sons, Ltd, pp. 55–110, doi:10.1002/9783527619542.ch2, ISBN 9783527619542
- ↑ Ely, Neal M.; Tsutsui, Minoru. (November 1975). "Organolanthanides and organoactinides. XV. Synthesis and properties of new .sigma.-bonded organolanthanide complexes". Inorganic Chemistry 14 (11): 2680–2687. doi:10.1021/ic50153a017. ISSN 0020-1669.
- ↑ 5.0 5.1 Werkema, Evan L. (2005). Synthesis, structure, and reactivity of bis(1,2,4-tri-t-butlcyclopentadienyl) complexes of cerium. OCLC 892838128.
- ↑ 6.0 6.1 6.2 Watson, Patricia L. (1983). "Facile C–H activation by lutetium–methyl and lutetium–hydride complexes". J. Chem. Soc., Chem. Commun. (6): 276–277. doi:10.1039/c39830000276. ISSN 0022-4936.
- ↑ 7.0 7.1 Molander, Gary A.; Romero, Jan Antoinette C. (2002-06-01). "Lanthanocene Catalysts in Selective Organic Synthesis". Chemical Reviews 102 (6): 2161–2186. doi:10.1021/cr010291+. ISSN 0009-2665. PMID 12059265.
- ↑ Evans, William J.; Grate, Jay W.; Doedens, Robert J. (March 1985). "Organolanthanide and organoyttrium hydride chemistry. 7. Reaction of the samarium-hydrogen bond in the organosamarium hydride [(C5Me5)2SmH]2 with carbon monoxide: formation, isomerization, and x-ray crystallographic characterization of the samarium complexes cis- and trans-{(C5Me5)2[(C6H5)3PO]Sm}2(.mu.-OCH:CHO)". Journal of the American Chemical Society 107 (6): 1671–1679. doi:10.1021/ja00292a034. ISSN 0002-7863.
- ↑ Schafer, Laurel L. (2017-06-12). "Organometallics—A Foundation for Catalysis Research". Organometallics 36 (11): 2053. doi:10.1021/acs.organomet.7b00390. ISSN 0276-7333.
- ↑ Weiss, Philip (October 1981). "Principles of polymerization, 2nd ed., George Odian, Wiley-Interscience, New York, 1981, 731 pp.". Journal of Polymer Science: Polymer Letters Edition 19 (10): 519. doi:10.1002/pol.1981.130191009. ISSN 0360-6384. Bibcode: 1981JPoSL..19..519W.
- ↑ Kaye, G.M. (October 1991). "Dear Sir". Journal of the Royal Society of Health 111 (5): 207. doi:10.1177/146642409111100520. ISSN 0264-0325. PMID 1795359.
- ↑ Heeres, H. J.; Heeres, A.; Teuben, J. H. (May 1990). "Organolanthanide-catalyzed cyclodimerizations of disubstituted alkynes". Organometallics 9 (5): 1508–1510. doi:10.1021/om00119a023. ISSN 0276-7333.
- ↑ "Front cover". Chemical Communications (16): 2053. 2009. doi:10.1039/b905898m. ISSN 1359-7345.
- ↑ Connolly, Michael L. (March 1985). "Computation of molecular volume". Journal of the American Chemical Society 107 (5): 1118–1124. doi:10.1021/ja00291a006. ISSN 0002-7863.
 |
