Chemistry:Sodium cyclopentadienide

| |||
| |||
| |||
 The cyclopentadienide anion
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Sodium cyclopentadienide | |||
| Other names
Sodium cyclopentadienylide, Cyclopentadienylsodium
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| C5H5Na | |||
| Molar mass | 88.085 g·mol−1 | ||
| Appearance | colorless solid | ||
| Density | 1.113 g/cm3 | ||
| decomposition | |||
| Solubility | THF | ||
| Hazards | |||
| Main hazards | flammable | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp− is the cyclopentadienide anion.[1] Sodium cyclopentadienide is a colorless solid, although samples often are pink owing to traces of oxidized impurities.[2]
Preparation
Sodium cyclopentadienide is commercially available as a solution in THF. It is prepared by treating cyclopentadiene with sodium:[3]
- 2 Na + 2 C
5H
6 → 2 NaC
5H
5 + H
2
The conversion can be conducted by heating a suspension of molten sodium in dicyclopentadiene.[2] In former times, the sodium was provided in the form of "sodium wire" or "sodium sand", a fine dispersion of sodium prepared by melting sodium in refluxing xylene and rapidly stirring.[4][5] Sodium hydride is a convenient base:[6]
- NaH + 2 C
5H
6 → NaC
5H
5 + H
2
In early work, Grignard reagents were used as bases. With a pKa of 15, cyclopentadiene can be deprotonated by many reagents.
Applications
Sodium cyclopentadienide is a common reagent for the preparation of metallocenes. For example, the preparation of ferrocene[4] and zirconocene dichloride:[7]
- 2 NaC
5H
5 + FeCl
2 → Fe(C
5H
5)
2 + 2 NaCl - ZrCl
4(thf)
2 + 2 NaCp → (C
5H
5)
2ZrCl
2 + 2 NaCl + 2 THF
Sodium cyclopentadienide is also used for the preparation of substituted cyclopentadienyl derivatives such as the ester and formyl derivatives:[8]
- NaC
5H
5 + O=C(OEt)
2 → NaC
5H
4CO
2Et + NaOEt
These compounds are used to prepare substituted metallocenes such as 1,1'-ferrocenedicarboxylic acid.[9]
Structure
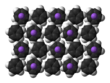
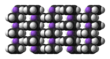
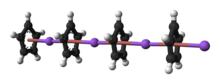
The nature of NaCp depends strongly on its medium and for the purposes of planning syntheses; the reagent is often represented as a salt Na+C5H−5. Crystalline solvent-free NaCp, which is rarely encountered, is a "polydecker" sandwich complex, consisting of an infinite chain of alternating Na+ centers sandwiched between μ-η5:η5-C5H5 ligands.[10] As a solution in donor solvents, NaCp is highly solvated, especially at the alkali metal as suggested by the isolability of the adduct Na(tmeda)Cp.[11]
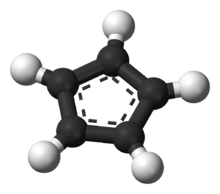
In contrast to alkali metal cyclopentadienides, tetrabutylammonium cyclopentadienide (Bu4N+C5H5−) was found to be supported entirely by ionic bonding and its structure is representative of the structure of the cyclopentadienide anion (C5H5−, Cp−) in the solid state. However, the anion deviates somewhat from a planar, regular pentagon, with C–C bond lengths ranging from 138.0 -140.1 pm and C–C–C bond angles ranging from 107.5-108.8°.[12]
See also
References
- ↑ Template:RedBookRef2005
- ↑ 2.0 2.1 Tarun K. Panda, Michael T. Gamer, Peter W. Roesky "An Improved Synthesis of Sodium and Potassium Cyclopentadienide" Organometallics, 2003, 22, 877–878.doi:10.1021/om0207865
- ↑ Cotton, F. Albert; Wilkinson, Geoffrey (1988), Advanced Inorganic Chemistry (5th ed.), New York: Wiley-Interscience, p. 139, ISBN 0-471-84997-9
- ↑ 4.0 4.1 Wilkinson, Geoffrey (1963). "Ferrocene". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv4p0473.; Collective Volume, 4, pp. 473
- ↑ Partridge, John J.; Chadha, Naresh K.; Uskokovic, Milan R. (1990). "An asymmetric hydroboration of 5-substituted cyclopentadienes: synthesis of methyl (1R,5R)-5-hydroxy-2-cyclopentene-1-acetate". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv7p0339.; Collective Volume, 7, pp. 339
- ↑ Girolami, G. S.; Rauchfuss, T. B.; Angelici, R. J. (1999). Synthesis and Technique in Inorganic Chemistry. CA: University Science Books: Mill Valley. ISBN 0935702482.
- ↑ Wilkinson, G.; Birmingham, J. G. (1954). "Bis-cyclopentadienyl Compounds of Ti, Zr, V, Nb and Ta". J. Am. Chem. Soc. 76 (17): 4281–84. doi:10.1021/ja01646a008.
- ↑ Macomber, D. W.; Hart, W. P.; Rausch, M. D. (1982). "Functionally Substituted Cyclopentadienyl Metal Compounds". Adv. Organomet. Chem.. Advances in Organometallic Chemistry 21: 1–55. doi:10.1016/S0065-3055(08)60377-9. ISBN 9780120311217.
- ↑ Petrov, Alex R.; Jess, Kristof; Freytag, Matthias; Jones, Peter G.; Tamm, Matthias (2013). "Large-Scale Preparation of 1,1′-Ferrocenedicarboxylic Acid, a Key Compound for the Synthesis of 1,1′-Disubstituted Ferrocene Derivatives". Organometallics 32 (20): 5946–5954. doi:10.1021/om4004972.
- ↑ Robert E. Dinnebier; Ulrich Behrens; Falk Olbrich (1997). "Solid State Structures of Cyclopentadienyllithium, -sodium, and -potassium. Determination by High-Resolution Powder Diffraction". Organometallics 16 (17): 3855–3858. doi:10.1021/om9700122.
- ↑ Elschenbroich, C. (2006). Organometallics. Wiley-VCH: Weinheim. ISBN 978-3-527-29390-2.
- ↑ Reetz, Manfred T.; Hütte, Stephan; Goddard, Richard (1995-03-01). "Tetrabutylammonium Salts of 2-Nitropropane, Cyclopentadiene and 9-Ethylfluorene: Crystal Structures and Use in Anionic Polymerization" (in en). Zeitschrift für Naturforschung B 50 (3): 415–422. doi:10.1515/znb-1995-0316. ISSN 1865-7117.
 |