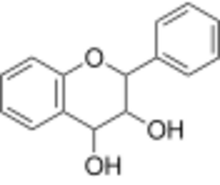
Chemistry:Leucoanthocyanidin
| |

| |
| Names | |
|---|---|
| IUPAC name
Flavan-3,4-diol
| |
| Systematic IUPAC name
2-Phenyl-3,4-dihydro-2H-1-benzopyran-3,4-diol | |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| UNII | |
| |
| Properties | |
| C15H14O3 | |
| Molar mass | 242.274 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Leucoanthocyanidin (flavan-3,4-diols) are colorless chemical compounds related to anthocyanidins and anthocyanins. Leucoanthocyanins can be found in Anadenanthera peregrina and in several species of Nepenthes including N. burbidgeae, N. muluensis, N. rajah, N. tentaculata, and N. × alisaputrana.[citation needed]
Such compounds include:
- Leucocyanidin
- Leucodelphinidin
- Leucofisetinidin
- Leucomalvidin
- Leucopelargonidin
- Leucopeonidin
- Leucorobinetinidin
- Melacacidin
- Teracacidin from Acacia obtusifolia and Acacia maidenii heartwoods[1]
Leucoanthocyanidins have been demonstrated to be intermediates in anthocyanidin biosynthesis in flowers of Matthiola incana.[2]
Bate-smith recommended in 1954 the use of the Forestal solvent for the isolation of leuco-anthocyanins.[3]
Metabolism
Leucoanthocyanidin dioxygenase uses flavan-3,4-diols to produce 3-hydroxyanthocyanidins.[4] The gene encoding the enzyme (PpLDOX) has been identified in peach[5] and expression has been studied in Vitis vinifera.[6]
References
- ↑ Clark-Lewis, JW; Dainis, I (1967). "Flavan derivatives. XIX. Teracacidin and isoteracacidin from Acacia obtusifolia and Acacia maidenii heartwoods; Phenolic hydroxylation patterns of heartwood flavonoids characteristic of sections and subsections of the genus Acacia". Australian Journal of Chemistry 20 (10): 2191–2198. doi:10.1071/CH9672191.
- ↑ Heller, Werner; Britsch, Lothar; Forkmann, Gert; Grisebach, Hans (1985). "Leucoanthocyanidins as intermediates in anthocyanidin biosynthesis in flowers of Matthiola incana R. Br.". Planta 163 (2): 191–196. doi:10.1007/BF00393505. PMID 24249337. https://doi.org/10.1007%2FBF00393505.
- ↑ Bate-SMITH, EC (September 1954). "Leuco-anthocyanins. 1. Detection and identification of anthocyanidins formed leuco-anthocyanins in plant tissues". Biochem. J. 58 (1): 122–5. doi:10.1042/bj0580122. PMID 13198862.
- ↑ "leucoanthocyanidin dioxygenase on mondofacto.com". http://www.mondofacto.com/facts/dictionary?leucoanthocyanidin+dioxygenase.
- ↑ Ogundiwin, E. A.; Peace, C. P.; Nicolet, C. M.; Rashbrook, V. K.; Gradziel, T. M.; Bliss, F. A.; Parfitt, D.; Crisosto, C. H. (2008). "Leucoanthocyanidin dioxygenase gene (PpLDOX): A potential functional marker for cold storage browning in peach". Tree Genetics & Genomes 4 (3): 543–554. doi:10.1007/s11295-007-0130-0. https://doi.org/10.1007%2Fs11295-007-0130-0.
- ↑ Regulation of the leucoanthocyanidin dioxygenase gene expression in Vitis vinifera, Gollop R; Farhi S.; Perl A. 2001
 |

