Chemistry:Leucopelargonidin
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
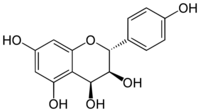
(2R,3S,4S)-Flavan-3,4,4′,5,7-pentol
| |
| Systematic IUPAC name
(2R,3S,4S)-2-(4-Hydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-3,4,5,7-tetrol | |
| Other names
(+)-Leucopelargonidin
cis-3,4-Leucopelargonidin Leucopelargonidine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H14O6 | |
| Molar mass | 290.271 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Leucopelargonidin is a colorless chemical compound related to leucoanthocyanins. It can be found in Albizia lebbeck (East Indian walnut), in the fruit of Anacardium occidentale (Cashew), in the fruit of Areca catechu (Areca nut), in the fruit of Hydnocarpus wightiana (Hindi Chaulmoogra), in the rhizome of Rumex hymenosepalus (Arizona dock), in Zea mays (Corn) and in Ziziphus jujuba (Chinese date).[1]
(+)-Leucopelargonidin can be synthesized from (+)-aromadendrin by sodium borohydride reduction.[2]
Metabolism
Dihydrokaempferol 4-reductase uses cis-3,4-leucopelargonidin and NADP+ to produce (+)-aromadendrin, NADPH, and H+.
Leucoanthocyanidin reductase transforms cis-3,4-leucopelargonidin into afzelechin.
References
- ↑ Leucopelargonidin on liberherbarum.com
- ↑ Heller, Werner; Britsch, Lothar; Forkmann, Gert; Grisebach, Hans (1985). "Leucoanthocyanidins as intermediates in anthocyanidin biosynthesis in flowers of Matthiola incana R. Br". Planta 163 (2): 191–196. doi:10.1007/BF00393505. PMID 24249337.
External links
 |

