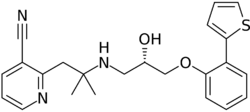
Chemistry:Lubabegron
 | |
| Clinical data | |
|---|---|
| Trade names | Experior |
| Other names | LY-488756 |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C29H29N3O3S |
| Molar mass | 499.63 g·mol−1 |
Lubabegron (trade name Experior) is a veterinary drug used to reduce ammonia emissions from animals and their waste.[1] Ammonia emissions are a concern in agricultural production because of detrimental effects on the environment, human health, and animal health.[2]
Lubabegron was approved by the U.S. Food and Drug Administration in 2018 for use in feedlot cattle.[3][4] It is the first drug approved for reducing ammonia emissions.[5] It is also approved for use in Canada.[6]
Lubabegron is a beta-adrenergic receptor agonist/antagonist.[7] The antagonist activity of lubabegron at β1 and β2 receptors prevents the stimulation of the β-AR found in the heart (β1) and trachea/bronchi (β2) of humans and, in doing so, avoids the potential negative side effects associated with β1 and β2 receptor activation. The β1-AR and β2-AR antagonist behavior of lubabegron could decrease lipolysis in adipose tissue, whereas the β3-AR agonist activity could increase skeletal muscle hypertrophy, possibly due to the differences in the second messenger systems and enzyme expression in skeletal muscle compared with adipose tissues.[8]
References
- ↑ "Drug reduces ammonia in cattle waste". American Veterinary Medical Association. December 31, 2018. https://www.avma.org/javma-news/2019-01-15/drug-reduces-ammonia-cattle-waste.
- ↑ "Ammonia Emission from Animal Feeding Operations and Its Impacts". Ohio State University Extension. May 16, 2014. https://ohioline.osu.edu/factsheet/AEX-723.1.
- ↑ "Effects of various doses of lubabegron on calculated ammonia gas emissions, growth performance, and carcass characteristics of beef cattle during the last 56 days of the feeding period". Translational Animal Science 5 (3): txab137. July 2021. doi:10.1093/tas/txab137. PMID 34532643.
- ↑ "Effects of feeding lubabegron on gas emissions, growth performance, and carcass characteristics of beef cattle housed in small-pen environmentally monitored enclosures during the last 3 mo of the finishing period". Journal of Animal Science 99 (12). December 2021. doi:10.1093/jas/skab338. PMID 34849995.
- ↑ "Advancing the Development of Innovative Veterinary Products". FDA. 5 October 2021. https://www.fda.gov/news-events/fda-voices/advancing-development-innovative-veterinary-products.
- ↑ "Lubabegron (LBGF) - Medicating Ingredient Brochure". Government of Canada. 22 January 2019. https://inspection.canada.ca/animal-health/livestock-feeds/medicating-ingredients/lubabegron/eng/1547583000099/1547583001862.
- ↑ "Freedom of Information Summary: Original New Animal Drug Application". https://animaldrugsatfda.fda.gov/adafda/app/search/public/document/downloadFoi/5005.
- ↑ "Comparison of beta-ligands used in cattle production: structures, safety, and biological effects". Journal of Animal Science 99 (8). August 2021. doi:10.1093/jas/skab094. PMID 34337648.
 |
