Chemistry:Lumisterol
From HandWiki
| |
| Names | |
|---|---|
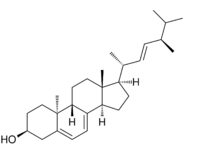
| IUPAC name
(22E)-9β,10α-Ergosta-5,7,22-trien-3β-ol
| |
| Systematic IUPAC name
(1R,3aR,7S,9aS,9bR,11aR)-1-[(2R,3E,5R)-5,6-Dimethylhept-3-en-2-yl]-9a,11a-dimethyl-2,3,3a,6,7,8,9,9a,9b,10,11,11a-dodecahydro-1H-cyclopenta[a]phenanthren-7-ol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C28H44O | |
| Molar mass | 396.659 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Lumisterol is a compound that is part of the vitamin D family of steroid compounds. It is the (9β,10α) stereoisomer of ergosterol and was produced as a photochemical by-product in the preparation of vitamin D1, which was a mixture of vitamin D2 and lumisterol.[1][2] Vitamin D2 can be formed from lumisterol by an electrocyclic ring opening and subsequent sigmatropic [1,7] hydride shift.
Lumisterol has an analog based on 7-dehydrocholesterol, known as lumisterol 3.[3]
References
- ↑ Dewick, Paul M. (2002). Medicinal Natural Products. A Biosynthetic Approach (Second ed.). New York: John Wiley & Sons. p. 259. ISBN 0-471-49640-5. http://nadjeeb.files.wordpress.com/2009/10/dewick-natural-prod.pdf.
- ↑ Friedmann, Ernst (1989). Neurath, Hans. ed. Vitamin D. Perspectives in Biochemistry. 1. Washington, DC: American Chemical Society. ISBN 978-0-8412-1621-1. https://books.google.com/books?id=q-Y8AAAAIAAJ&pg=PA247.
- ↑ "Lumisterol 3 (CID=111049)" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/lumisterol_3. Retrieved 10 April 2018.
 |

