Chemistry:Magnesium formate
From HandWiki
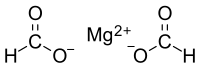
| |
| Names | |
|---|---|
| IUPAC name
Magnesium diformate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| Mg(HCO2)2 | |
| 14 g/100g at 0 °C 14.4 g/100g at 20 °C | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Magnesium formate is a magnesium salt of formic acid. It is an inorganic compound. It consists of a magnesium cation and formate anion. It can be prepared by reacting magnesium oxide with formic acid. The dihydrate is formed when crystallizing from the solution. The dihydrate dehydrates at 105 °C to form anhydrate, then decomposes at 500 °C to produce magnesium oxide.[1] Magnesium formate can be used for organic syntheses.[2]
References
- ↑ D. Dollimore, J.P. Gupta, D.V. Nowell (May 1979). "The thermal decomposition of metal formates. II. Solid state thermal decomposition studies on magnesium formate dihydrate". Thermochimica Acta 30 (1–2): 339–350. doi:10.1016/0040-6031(79)85069-8. ISSN 0040-6031. http://linkinghub.elsevier.com/retrieve/pii/0040603179850698. Retrieved 2018-08-09.
- ↑ Brückner, Reinhard; Zettlmeier, Wolfgang (2015) (in de). Reaktionsmechanismen organische Reaktionen, Stereochemie, moderne Synthesemethoden. Berlin. p. 316. ISBN 978-3-662-45683-5. OCLC 901537772.
 |
