Chemistry:Maillard reaction

The Maillard reaction (/maɪˈjɑːr/ my-YAR; French: [majaʁ]) is a chemical reaction between amino acids and reducing sugars to create melanoidins, the compounds which give browned food its distinctive flavor. Seared steaks, fried dumplings, cookies and other kinds of biscuits, breads, toasted marshmallows, and many other foods undergo this reaction. It is named after French chemist Louis Camille Maillard, who first described it in 1912 while attempting to reproduce biological protein synthesis.[1][2] The reaction is a form of non-enzymatic browning which typically proceeds rapidly from around 140 to 165 °C (280 to 330 °F). Many recipes call for an oven temperature high enough to ensure that a Maillard reaction occurs.[3] At higher temperatures, caramelization (the browning of sugars, a distinct process) and subsequently pyrolysis (final breakdown leading to burning and the development of acrid flavors) become more pronounced.[4]
The reactive carbonyl group of the sugar reacts with the nucleophilic amino group of the amino acid and forms a complex mixture of poorly characterized molecules responsible for a range of aromas and flavors. This process is accelerated in an alkaline environment (e.g., lye applied to darken pretzels; see lye roll), as the amino groups (RNH+
3 → RNH
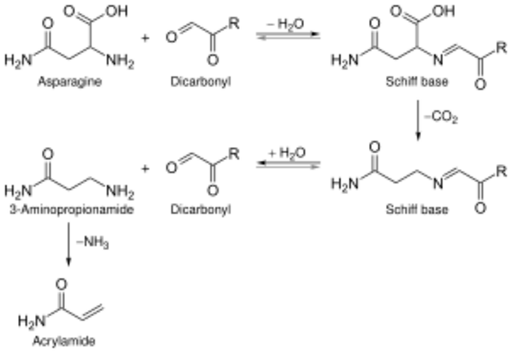
2) are deprotonated, and hence have an increased nucleophilicity. This reaction is the basis for many of the flavoring industry's recipes. At high temperatures, a probable[5] carcinogen called acrylamide can form.[6] This can be discouraged by heating at a lower temperature, adding asparaginase, or injecting carbon dioxide.[5]
In the cooking process, Maillard reactions can produce hundreds of different flavor compounds depending on the chemical constituents in the food, the temperature, the cooking time, and the presence of air. These compounds, in turn, often break down to form yet more flavor compounds. Flavour scientists have used the Maillard reaction over the years to make artificial flavors.
History
In 1912, Louis Camille Maillard published a paper describing the reaction between amino acids and sugars at elevated temperatures.[1] In 1953, chemist John E. Hodge with the U.S. Department of Agriculture established a mechanism for the Maillard reaction.[7][8]
Foods and products

The Maillard reaction is responsible for many colors and flavors in foods, such as the browning of various meats when seared or grilled, the browning and umami taste in fried onions and coffee roasting. It contributes to the darkened crust of baked goods, the golden-brown color of French fries and other crisps, browning of malted barley as found in malt whiskey and beer, and the color and taste of dried and condensed milk, dulce de leche, toffee, black garlic, chocolate, toasted marshmallows, and roasted peanuts.[citation needed]
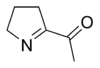
6-Acetyl-2,3,4,5-tetrahydropyridine is responsible for the biscuit or cracker-like flavor present in baked goods such as bread, popcorn, and tortilla products. The structurally related compound 2-acetyl-1-pyrroline has a similar smell and also occurs naturally without heating. The compound gives varieties of cooked rice and the herb pandan (Pandanus amaryllifolius) their typical smells. Both compounds have odor thresholds below 0.06 nanograms per liter.[9]

The browning reactions that occur when meat is roasted or seared are complex and occur mostly by Maillard browning[10] with contributions from other chemical reactions, including the breakdown of the tetrapyrrole rings of the muscle protein myoglobin. Maillard reactions also occur in dried fruit.[11]
Caramelization is an entirely different process from Maillard browning, though the results of the two processes are sometimes similar to the naked eye (and taste buds). Caramelization may sometimes cause browning in the same foods in which the Maillard reaction occurs, but the two processes are distinct. They are both promoted by heating, but the Maillard reaction involves amino acids, whereas caramelization is the pyrolysis of certain sugars.[12]
In making silage, excess heat causes the Maillard reaction to occur, which reduces the amount of energy and protein available to the animals that feed on it.[13]
Archaeology
In archaeology, the Maillard process occurs when bodies are preserved in peat bogs. The acidic peat environment causes a tanning or browning of skin tones and can turn hair to a red or ginger tone. The chemical mechanism is the same as in the browning of food, but it develops slowly over time due to the acidic action on the bog body. It is typically seen on Iron Age bodies and was described by Painter in 1991 as the interaction of anaerobic, acidic, and cold (typically 4 °C (39 °F)) sphagnum acid on the polysaccharides.
The Maillard reaction also contributes to the preservation of paleofeces.[14]
Chemical mechanism
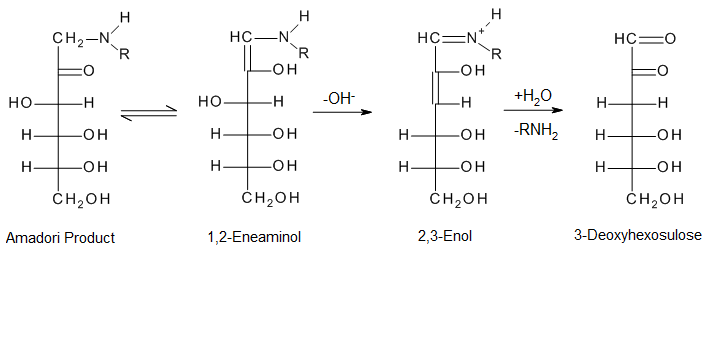
- The carbonyl group of the sugar reacts with the amino group of the amino acid, producing N-substituted glycosylamine and water
- The unstable glycosylamine undergoes Amadori rearrangement, forming ketosamines
- Several ways are known for the ketosamines to react further:
- Produce two water molecules and reductones
- Diacetyl, pyruvaldehyde, and other short-chain hydrolytic fission products can be formed.
- Produce brown nitrogenous polymers and melanoidins
The open-chain Amadori products undergo further dehydration and deamination to produce dicarbonyls.[15] This is a crucial intermediate.
Dicarbonyls react with amines to produce Strecker aldehydes through Strecker degradation.[16]
Acrylamide, a possible human carcinogen,[17] can be generated as a byproduct of Maillard reaction between reducing sugars and amino acids, especially asparagine, both of which are present in most food products.[18][19]
See also
- Akabori amino-acid reaction
- Advanced glycation end-product
- Baking
- Caramelization
- Script error: The function "transl" does not exist.
References
- ↑ 1.0 1.1 Maillard, L. C. (1912). "Action des acides amines sur les sucres; formation de melanoidines par voie méthodique" (in fr). Comptes Rendus 154: 66–68. https://babel.hathitrust.org/cgi/pt?id=umn.31951d00015504w&view=1up&seq=74.
- ↑ Chichester, C. O., ed (1986). Advances in Food Research. Advances in Food and Nutrition Research. 30. Boston: Academic Press. p. 79. ISBN 0-12-016430-2.
- ↑ Bui, Andrew (2017-09-29). "Why So Many Recipes Call for a 350-Degree Oven". https://www.tastingtable.com/cook/national/why-bake-350-degrees.
- ↑ "Here's How to Sear a Steak to Perfection" (in en). 2021-09-06. https://homecookworld.com/how-to-sear-a-steak/.
- ↑ 5.0 5.1 Tamanna, N; Mahmood, N (2015). "Food Processing and Maillard Reaction Products: Effect on Human Health and Nutrition.". International Journal of Food Science 2015: 526762. doi:10.1155/2015/526762. ISSN 2314-5765. PMID 26904661.
- ↑ 6.0 6.1 Tareke, E.; Rydberg, P.; Karlsson, Patrik; Eriksson, Sune; Törnqvist, Margareta (2002). "Analysis of acrylamide, a carcinogen formed in heated foodstuffs". J. Agric. Food Chem. 50 (17): 4998–5006. doi:10.1021/jf020302f. PMID 12166997.
- ↑ Hodge, J. E. (1953). "Dehydrated Foods, Chemistry of Browning Reactions in Model Systems". Journal of Agricultural and Food Chemistry 1 (15): 928–43. doi:10.1021/jf60015a004.
- ↑ Everts, Sarah (October 1, 2012). "The Maillard Reaction Turns 100". Chemical & Engineering News 90 (40): 58–60. doi:10.1021/cen-09040-scitech2. http://cen.acs.org/articles/90/i40/Maillard-Reaction-Turns-100.html.
- ↑ Harrison, T. J.; v, G. R. (2005). "An expeditious, high-yielding construction of the food aroma compounds 6-acetyl-1,2,3,4-tetrahydropyridine and 2-acetyl-1-pyrroline". J. Org. Chem. 70 (26): 10872–74. doi:10.1021/jo051940a. PMID 16356012.
- ↑ McGee, Harold (2004). On Food and Cooking: The Science and Lore of the Kitchen. New York: Scribner. pp. 778–79. ISBN 978-0-684-80001-1.
- ↑ Miranda, Gonzalo; Berna, Angel; Mulet, Antonio (4 February 2019). "Dried-Fruit Storage: An Analysis of Package Headspace Atmosphere Changes". Foods 8 (2): 56. doi:10.3390/foods8020056. PMID 30720722.
- ↑ Krystal, Becky (2020-01-31). "The Maillard reaction: What it is and why it matters" (in en-US). The Washington Post. https://www.washingtonpost.com/news/voraciously/wp/2020/01/31/the-maillard-reaction-what-it-is-and-why-it-matters/.
- ↑ Cooper, Phil (2 May 2017). "Grass Silage Stability and Maillard Silage" (in en). https://www.fcgagric.com/2017/05/02/grass-silage-stability-and-maillard-silage/.
- ↑ Dove, Alan (11 February 2016). "Hard-core sequencing". American Association for the Advancement of Science. https://www.science.org/content/article/hard-core-sequencing. "In a dry environment, the Maillard reaction—the same chemical process that browns a steak—causes feces to develop a protective outer shell."
- ↑ Nursten, Harry (2007). "The Chemistry of Nonenzymic Browning". The Maillard Reaction. pp. 5–30. doi:10.1039/9781847552570-00005. ISBN 978-0-85404-964-6.
- ↑ Stadler, Richard H.; Robert, Fabien; Riediker, Sonja; Varga, Natalia; Davidek, Tomas; Devaud, Stéphanie; Goldmann, Till; Hau, Jörg et al. (August 2004). "In-Depth Mechanistic Study on the Formation of Acrylamide and Other Vinylogous Compounds by the Maillard Reaction". Journal of Agricultural and Food Chemistry 52 (17): 5550–5558. doi:10.1021/jf0495486. PMID 15315399.
- ↑ Acrylamide. Cancer.org. Retrieved on 2016-07-24.
- ↑ Virk-Baker, Mandeep K.; Nagy, Tim R.; Barnes, Stephen; Groopman, John (29 May 2014). "Dietary Acrylamide and Human Cancer: A Systematic Review of Literature". Nutrition and Cancer 66 (5): 774–790. doi:10.1080/01635581.2014.916323. PMID 24875401.
- ↑ Mottram, Donald S.; Wedzicha, Bronislaw L.; Dodson, Andrew T. (October 2002). "Acrylamide is formed in the Maillard reaction". Nature 419 (6906): 448–449. doi:10.1038/419448a. PMID 12368844. Bibcode: 2002Natur.419..448M.
Further reading
- Van Soest, Peter J. (1982). Nutritional Ecology of the Ruminant (2nd ed.). Ithaca, NY: Cornell University Press. ISBN:9780801427725. OCLC 29909839.
External links
 |