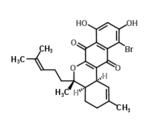
Chemistry:Marinone
| |
| Names | |
|---|---|
| IUPAC name
(4aR,5S,12bS)-11-bromo-8,10-dihydroxy-2,5-dimethyl-5-(4-methylpent-3-enyl)-3,4,4a,12b-tetrahydronaphtho[2,3-c]isochromene-7,12-dione
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C25H27BrO5 | |
| Molar mass | 487.390 g·mol−1 |
| Related compounds | |
Related compounds
|
Debromomarinone |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Marinone is an antibiotic made by marine actinomycetes.[1]
Biosynthesis
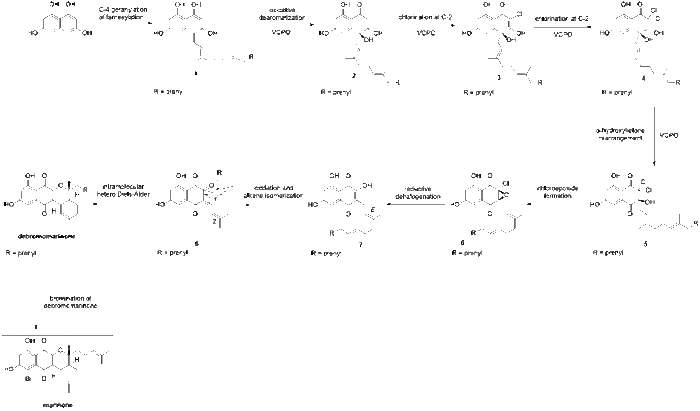
The proposed biosynthesis of marinone was first reported by Murray et al. (2018).[2] The biosynthesis of marinone begins with 1,3,6,8-tetrahydroxynaphthalene (THN), which is known to be biosynthesized via the condensation of five malonyl-coenzyme A units followed by the aromatization of the resulting pentaketide using a type III polyketide synthase.[3] Next, THN undergoes geranylation or farnesylation at the C-4 position, yielding 1 (Fig. 1). This transformation is catalyzed in vivo by NphB aromatic prenyltransferase in naphterpin biosynthesis[4] or by CnqP3 or CnqP4 in marinone biosynthesis.[5] Then, 1 undergoes oxidative dearomatization which is catalyzed by VCPO, which is a vanadium-dependent chloroperoxidase enzyme. This transformation yields compound 2. Compound 2 then undergoes two consecutive chlorinations at the C2 position, catalyzed by VCPO, to yield 4. Next, a VCPO catalyzed α-hydroxyketone rearrangement shifts the geranyl substituent from C-4 to C-3, yielding 5. Exposure of 5 to mildly basic conditions induces cyclization to yield the α-chloroepoxide, 6. This is followed by the reductive halogenation of the α-chloroepoxide to yield the hydroxynaphthoquinone, 7. Next, oxidation at the C-2 position and facile E/Z isomerization of the double bond affords the enone, 8, which undergoes a intramolecular hetero-Diels-Alder to yield debromomarinone. Lastly, the vanadium-dependent bromoperoxidase catalyzes the bromination of debromomarinone at the C-5 position to result in the formation of marinone.
References
- ↑ "Marinone and debromomarinone: Antibiotic sesquiterpenoid naphthoquinones of a new structure class from a marine bacterium" (in en). Tetrahedron Letters 33 (50): 7663–7666. December 1992. doi:10.1016/0040-4039(93)88010-G.
- ↑ 2.0 2.1 "Total Synthesis Establishes the Biosynthetic Pathway to the Naphterpin and Marinone Natural Products". Angewandte Chemie 57 (34): 11009–11014. August 2018. doi:10.1002/anie.201804351. PMID 29935040.
- ↑ "Naphthoquinone-Based Meroterpenoids from Marine-Derived Streptomyces sp. B9173". Biomolecules 10 (8): 1187. August 2020. doi:10.3390/biom10081187. PMID 32824158.
- ↑ "Structural basis for the promiscuous biosynthetic prenylation of aromatic natural products". Nature 435 (7044): 983–987. June 2005. doi:10.1038/nature03668. PMID 15959519. Bibcode: 2005Natur.435..983K.
- ↑ "Studies on terpenoids produced by actinomycetes. 5-dimethylallylindole-3-carboxylic Acid and A80915G-8"-acid produced by marine-derived Streptomyces sp. MS239". The Journal of Antibiotics 61 (2): 75–80. February 2008. doi:10.1038/ja.2008.113. PMID 18408326.
External links
 |

