Chemistry:Melamine cyanurate
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,3,5-Triazinane-2,4,6-trione—1,3,5-triazine-2,4,6-triamine (1/1) | |
| Other names
Melamine–cyanuric acid compound, melamine–cyanuric acid adduct, melamine cyanurate, melamine isocyanurate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| MeSH | melamine+cyanurate |
PubChem CID
|
|
| |
| |
| Properties | |
| C6H9N9O3
(C3H6N6·C3H3N3O3) | |
| Molar mass | 255.19 g/mol |
| none | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
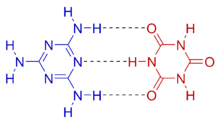
Melamine cyanurate, also known as melamine–cyanuric acid adduct or melamine–cyanuric acid complex, is a crystalline complex formed from a 1:1 mixture of melamine and cyanuric acid. The substance is not a salt despite its non-systematic name melamine cyanurate. The complex is held together by an extensive two-dimensional network of hydrogen bonds between the two compounds, reminiscent of the guanine–cytosine base pairs found in DNA.[2] Melamine cyanurate forms spoke-like crystals from aqueous solutions [3] and has been implicated as a causative agent for toxicity seen in the Chinese protein export contamination and the 2007 pet food recall.[3]
Chemistry
The substance is best described as a melamine-cyanuric acid complex, or non-covalent adduct. The two compounds do not form a salt as suggested by its colloquial name melamine cyanurate.
Uses
Melamine cyanurate is used as a flame retardant, most commonly in polybutylene terephthalate (PBT), polyamide 6 (nylon 6) and polyamide 6,6 (nylon 6:6).[4] It is also used to fireproof in polyester fabrics.
Toxicity
It has been considered to be more toxic than either melamine or cyanuric acid alone.[5]
-1">50 in rats and mice (ingested):
- 4.1 g/kg – Melamine cyanurate
- 6.0 g/kg – Melamine[clarification needed]
- 7.7 g/kg – Cyanuric acid
A toxicology study conducted after recent pet food recalls concluded that the combination of melamine and cyanuric acid in diet does lead to acute kidney injury in cats.[6] A 2008 study produced similar experimental results in rats and characterized the melamine and cyanuric acid in contaminated pet food from the 2007 outbreak.[7]
See also
- 1,3,5-Triazine
- Hydrogen bonding
References
- ↑ EPA: Substance :
- ↑ "Surface self-assembly of the cyanuric acid-melamine hydrogen bonded network". Chem. Commun. (5): 538–540. 2006. doi:10.1039/b514389f. PMID 16432575.
- ↑ 3.0 3.1 Lili He; Yang Liu; Mengshi Lin; Joseph Awika; David R Ledoux; Hao Li; Azlin Mustapha (2008). "A new approach to measure melamine, cyanuric acid, and melamine cyanurate using surface enhanced Raman spectroscopy coupled with gold nanosubstrates". Sens. & Instrumen. Food Qual. 2: 66–71. doi:10.1007/s11694-008-9038-0.
- ↑ Gijsman, Pieter; Steenbakkers, Rieky; Fürst, Christian; Kersjes, Joyce (2002). "Differences in the flame retardant mechanism of melamine cyanurate in polyamide 6 and polyamide 66". Polymer Degradation and Stability 78 (2): 219–224. doi:10.1016/S0141-3910(02)00136-2.
- ↑ A.A. Babayan, A.V.Aleksandryan, "Toxicological characteristics of melamine cyanurate, melamine and cyanuric acid", Zhurnal Eksperimental'noi i Klinicheskoi Meditsiny, Vol.25, 345-9 (1985). Original article in Russian.
- ↑ Puschner et al. (November 2007). Assessment of melamine and cyanuric acid toxicity in cats. Journal of Veterinary Diagnostic Investigation. Retrieved on 2007-11-16.
- ↑ Dobson (August 2008). "Identification and characterization of toxicity of contaminants in pet food leading to an outbreak of renal toxicity in cats and dogs.". Toxicological Sciences 106 (1): 251–62. doi:10.1093/toxsci/kfn160. PMID 18689873.
 |


