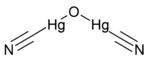
Chemistry:Mercury oxycyanide
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
cyano(cyanomercuriooxy)mercury
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C2Hg2N2O | |
| Molar mass | 469.219 g·mol−1 |
| Density | 5.94 |
| Structure[1] | |
| orthorhombic | |
| Pnam | |
a = 18.93, b = 7.09, c = 3.90
| |
Formula units (Z)
|
4 |
| rough V | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Mercury oxycyanide is a chemical compound, an organomercury derivative. It is both explosive and highly toxic, producing symptoms of both mercury and cyanide poisoning following exposure.[2][3] left|thumb|Old mercury oxycyanide jar
See also
References
- ↑ "The crystal structure of mercury(II) oxycyanide". Zeitschrift für Kristallographie - Crystalline Materials 118 (1–6). January 1963. doi:10.1524/zkri.1963.118.16.248.
- ↑ "The determination of mercury oxycyanide". The Journal of Pharmacy and Pharmacology 10 (7): 442–6. July 1958. doi:10.1111/j.2042-7158.1958.tb10326.x. PMID 13564415.
- ↑ "Mercury oxycyanide and mercuric cyanide poisoning: two cases". Intensive Care Medicine 21 (12): 1051–3. December 1995. doi:10.1007/BF01700673. PMID 8750135.
 |

